Hydrogen is the lightest and most abundant element in the universe, and it has many important chemical and physical properties. Hydrogen is a diatomic gas at standard temperature and pressure (STP), which means that its molecules consist of two hydrogen atoms bonded together.
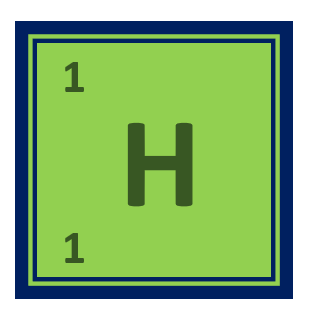
Physical properties of Hydrogen element:
- At room temperature, hydrogen is a colorless, odorless, tasteless, and extremely combustible gas.
- It is the lightest gas, with a density roughly 14 times that of air.
- Although hydrogen gas is highly reactive, it easily forms compounds with other elements.
Table of Physical properties of hydrogen:
| Property | Description |
| Chemical symbol | H |
| Atomic number | 1 |
| Atomic mass | 1.008 amu |
| Phase at STP | Gas (diatomic) |
| Color | Colorless |
| Odor | Odorless |
| Density | 0.0899 g/L (at STP) |
| Solubility | Slightly soluble in water, highly soluble in organic solvents |
| Melting point | -259.14°C |
| Boiling point | -252.87°C |
| Triple point | -259.34°C at 7.042 kPa |
| Critical point | -240.17°C at 1.293 MPa |
| Thermal conductivity | 0.1805 W/(m·K) at 25°C |
| Specific heat capacity | 28.836 J/(mol·K) at 25°C |
| Viscosity | 8.76 × 10^(-6) Pa·s at 0°C |
Chemical properties of Hydrogen element:
- Hydrogen has a valency of +1 and may combine with other elements to generate both ionic and covalent molecules.
- It combines with oxygen to make water, a highly exothermic reaction that can be utilized to generate energy.
- Many other elements, including halogens, nitrogen, sulfur, and carbon, react with hydrogen gas.
- It can act as a reducing agent, donating electrons when it reacts with oxidizing substances.
Table of Chemical properties of hydrogen:
| Property | Description |
| Chemical symbol | H |
| Atomic number | 1 |
| Atomic mass | 1.008 amu |
| Reactivity | Hydrogen is a highly reactive element, combining readily with many other elements, especially oxygen |
| Flammability | Hydrogen is highly flammable and can ignite easily in air |
| Oxidation states | -1 (hydride), +1 (H) |
| Acid-base properties | Hydrogen gas can act as either an acid or a base, depending on the chemical reaction |
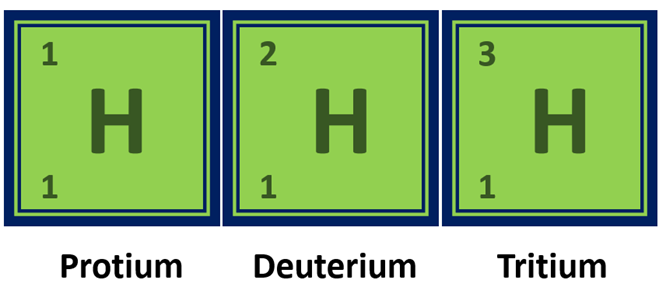
| Isotopes | Protium (1H), Deuterium (2H), Tritium (3H) |
Valency of Hydrogen element:
Hydrogen has a valency of +1, which means it can form one chemical bond with other elements. It can also form two chemical bonds in some cases, such as in H2, the diatomic form of hydrogen.
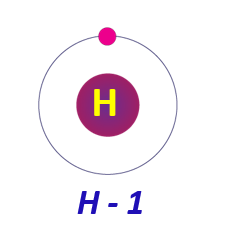
Electronic configuration of Hydrogen:
Hydrogen has an electrical configuration of 1s1, which implies it contains one electron in the first energy level. It can either give or share its electron to form a cation or a covalent bond.
Electronic configuration of Hydrogen in a diagram
Isotopes of Hydrogen
Hydrogen has three naturally occurring isotopes: protium, deuterium, and tritium, which have different numbers of neutrons in their nuclei.
Isotopes of Hydrogen element in a diagram
Uses of Hydrogen:
- Hydrogen is utilized in the manufacture of ammonia, a vital component of fertilizers.
- It is also utilized in the manufacture of methanol, an essential industrial chemical.
- Hydrogen gas can be utilized as a vehicle fuel, offering an alternative to gasoline and diesel.
- It is used in the semiconductor and aircraft industries because it has a high specific energy and can be stored efficiently.
Reactions with other elements:
In a highly exothermic reaction, hydrogen gas easily combines with oxygen to generate water.
It can also react with halogens to produce hydrogen halides, sulfur to produce hydrogen sulfide, and carbon to produce methane and other hydrocarbons.
Industrial uses of Hydrogen:
Chemicals like ammonia, methanol, and hydrogen peroxide can all be made from hydrogen.
In addition to being a fuel in fuel cells, it is also utilized in the manufacturing of metals and semiconductors.
Medical uses of Hydrogen:
Certain medical diseases, such as respiratory distress syndrome and bronchopulmonary dysplasia in premature infants, are treated with hydrogen gas.
It may also have therapeutic potential for various illnesses because it acts as an antioxidant and reduces inflammation.
Facts about Hydrogen based compounds
Hydrogen peroxide in ear
Hydrogen peroxide is a common household disinfectant that is also used in medicine for a variety of purposes, including wound cleaning and disinfection and earwax removal. Hydrogen peroxide, when used correctly, can help to break down and dissolve earwax buildup, leaving the ear canal clean and clear.
How to use hydrogen peroxide in ear
Follow these instructions to utilize hydrogen peroxide in the ear:
Buy 3% hydrogen peroxide from a pharmacy or medicine shop.
Tilt your head to one side and turn your ear upward.
Fill your ear canal with few drops of hydrogen peroxide using an eyedropper or a small syringe.
Hold this position for up to 2 minutes to allow the hydrogen peroxide to dissolve the earwax.
When the timer goes off, tilt your head in the opposite direction to allow the hydrogen peroxide and any loosened earwax to drain out of your ear.
To remove any excess hydrogen peroxide, gently wipe the outer ear with a clean towel or tissue.
Hydrogen bonds definition
Hydrogen bonds are a sort of chemical bond formed when a hydrogen atom covalently bound to an electronegative atom (such as nitrogen, oxygen, or fluorine) is attracted to another electronegative atom in a different molecule or part of the same molecule. When a partially positive hydrogen atom attracts a partially negative atom of another molecule or another part of the same molecule, a hydrogen bond is formed.
Hydrogen bonds are relatively weak compared to covalent bonds, but they are important in many biological processes, such as the structure of proteins, DNA, and RNA. Hydrogen bonds also play a role in the properties of water and other molecules. The strength of a hydrogen bond depends on the distance between the hydrogen and the electronegative atom, as well as the angle of the bond.
Hydrogen bonds in DNA
Hydrogen bonding are essential to the structure of DNA (deoxyribonucleic acid). DNA is made up of a series of nucleotides, which are the molecule’s building blocks. Each nucleotide is made up of three parts: a sugar molecule, a phosphate group, and a nitrogenous base. Adenine (A), thymine (T), cytosine (C), and guanine (G) are nitrogenous bases found in DNA.
Hydrogen bonds hold the nitrogenous bases of DNA together. In particular, A and C always pair with T and G, respectively. The nitrogenous bases are positioned in such a way that the hydrogen atoms of A and T, as well as the hydrogen atoms of C and G, are close enough to form hydrogen bonds between them. The hydrogen bonds are relatively weak, but when there are so many of them between the nitrogenous bases, they help to stabilize the double helix structure of the DNA molecule.
The two strands of DNA run in opposite directions and are bound together by hydrogen bonds. By joining the nitrogenous bases, hydrogen bonds help to hold the two strands together. The hydrogen bonds between the nitrogenous bases are also crucial for complementary base pairing, which enables for DNA replication by separating the two strands and using each strand as a template for the synthesis of a new complementary strand.
Which enzyme breaks the hydrogen bonds during replication?
The enzyme that breaks the hydrogen bonds between the complementary nitrogenous bases during DNA replication is called DNA helicase. DNA helicase is a type of enzyme that unwinds the double helix structure of DNA by breaking the hydrogen bonds between the nitrogenous bases.
The double-stranded DNA molecule is unraveled by DNA helicase at the replication fork, resulting in two single strands of DNA. The split single strands are then used as templates to generate new complementary strands. The hydrogen bonding between the nitrogenous bases causes complementary base pairing of the new strands, which is aided by other enzymes such as DNA polymerase.
What compounds are made from hydrogen?
Hydrogen is a highly reactive element and can form compounds with a wide range of other elements. Some common compounds that are made from hydrogen include:
Water (H2O): This is the most common compound made from hydrogen. Water is a molecule made up of two hydrogen atoms and one oxygen atom, and it is essential for life on Earth.
Hydrogen peroxide (H2O2): This is a chemical compound composed of two hydrogen atoms and two oxygen atoms.
Methane (CH4): This is a compound made up of one carbon atom and four hydrogen atoms.
Ammonia (NH3): This is a compound made up of one nitrogen atom and three hydrogen atoms.
Hydrochloric acid (HCl): This is a compound made up of one hydrogen atom and one chlorine atom.
Is hydrogen a metal or nonmetal?
Hydrogen is not a metal. It has an atomic number of one and is the first element in the periodic table. Although hydrogen is in group 1 of the periodic table, which includes the alkali metals, it is not a metal.
Can hydrogen be a metal?
In concept, hydrogen might exhibit metallic qualities and be classified as a metal under specific conditions. However, this has yet to be observed experimentally, and scientists continue to question whether metallic hydrogen can exist at all.
Hydrogen is predicted to undergo a phase transition at extremely high pressures, such as those seen in the cores of gas giant planets like Jupiter, in which its characteristics change substantially. Theoretical simulations show that at pressures of roughly 4 million atmospheres, hydrogen might transform into a metallic solid with metal-like characteristics.
Creating these circumstances on Earth, however, is extremely difficult, and no one has yet definitively demonstrated the existence of metallic hydrogen. Some experiments claimed to have generated metallic hydrogen, but the results were contentious and were not widely recognized by the scientific community.


