Introduction
Chemical solutions in the laboratory and day-to-day use can be either acidic, basic / alkali, or neutral. But it is difficult to identify the ph of solutions just by observing. Most of the time the solutions are colorless.
A chemical indicator when added to a solution gives a distinct color for its acidic or basic property. Usually, chemical indicators or else ph indicators have two different colors
Natural indicators
Natural indicators are found in a number of plants. Extracting the chemical indicator from the parts of the plant can be difficult sometimes. Red cabbage and Hibiscus are common examples for natural indicators. The dye that acts as a natural indicator can be extracted from red cabbage by distillation.
Red Cabbage

Hibiscus flower

Chemical indicators used in laboratories
Common chemical indicators used in the laboratory are discussed in this article. They are;
- Phenolphthalein
- Methyl Orange
- Litmus papers
- pH papers
Phenolphthalein indicator and its action
Phenolphthalein is a commonly used indicator in the laboratory for acid-base titrations. It was made by the German scientist Adolf von Baeyer. Phenolphthalein is one of the phthalein dyes used in chemistry. Phenolphthalein comes as a white powder that is soluble in alcohol.
Phenolphthalein has a distinct pink color in basic solutions and it is colorless in acids.
This color change occurs around pH 8.3 to 10.0.
Phenolphthalein is also used in color-changing toys, for recreational activities such as writing invisible messages, and in concrete work where carbonation is analyzed.
Methyl orange indicator and its action
Methyl orange is commonly used in acid-base titrations since it shows a clear change of colors from yellow to red/orange. In acids methyl, orange has a red/orange color whereas in basic (alkali) solutions methyl orange becomes yellowish.
This color change occurs around pH 3.1 to 4.4.
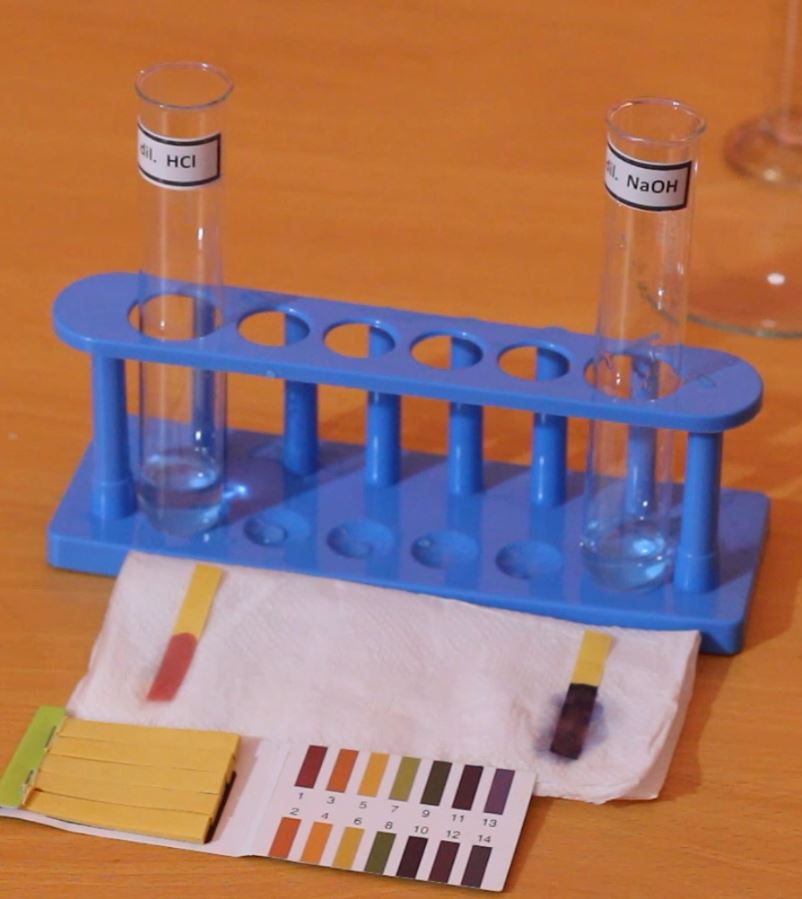
Litmus papers
Litmus papers are made from dyes extracted from specific lichens. The dyes are absorbed in papers. Litmus papers are commercially available as red litmus and blue litmus.
They are commonly used as they have a distinct color change for acids and alkali.
Blue litmus turns into red in acids. Blue litmus stays blue in alkali and water.
Red litmus changes its color into blue in alkali solutions. Red litmus stays red in acids and water.
Therefore both litmus papers are used together in practical situations (because alkali solutions are identified by blue litmus and acidic solutions are not identified by red litmus)
The red color is produced under pH 4.5 (acid). The blue color is produced above pH 8.3 (alkali)
Litmus papers soaked in water can be used to identify gasses emitted from chemical reactions. This occurs when gasses are dissolved in the water in the wet litmus papers.
pH papers
pH papers are made by the addition of different chemical indicators that have distinct colors in each pH value. The solution is absorbed into the paper and is mostly made from a mixture of weak acids. Usually, pH papers show a reddish color in acidic solutions and a greenish-blue in alkali solutions.
pH paper comes in a set of small papers and a color guide to compare the strip to read the pH value. When checking for pH value, one strip of pH paper needs to be dipped into the chemical solution that is needed to be checked and then the color change of the strip needs to be compared with the reference colors provided by the manufacturer. There are usually different shades for each pH value.
Conclusion
Chemical indicators show us a distinct color change in chemical solutions. It eases the identification of acids and alkali. Natural and artificial chemical indicators are used. Having basic knowledge in pH values and chemical indicators is useful for titrations and studying chemical reactions.