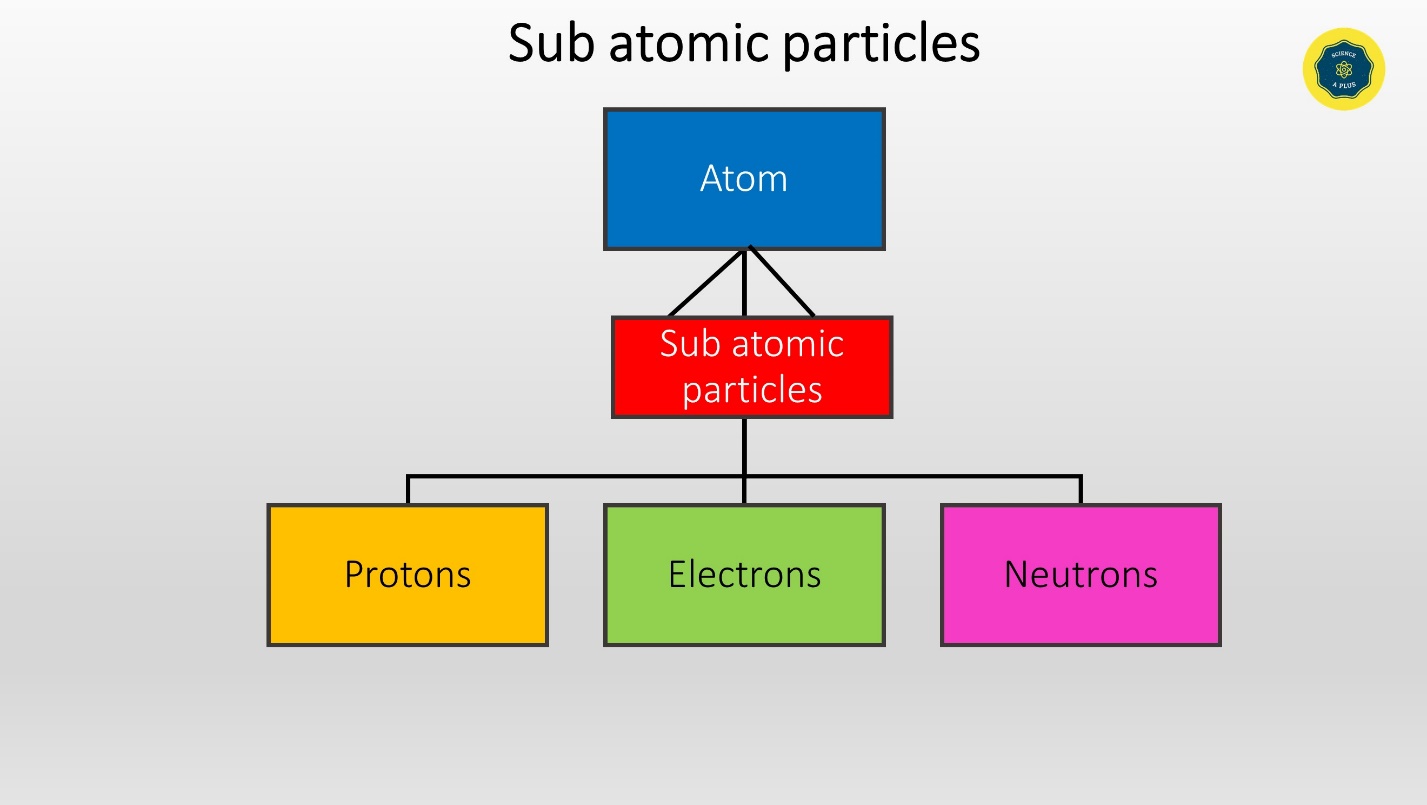
Atom is the building unit of matter. Atoms are made of sub atomic particles. The sub atomic particles are
- Protons
- Electrons
- Neutrons
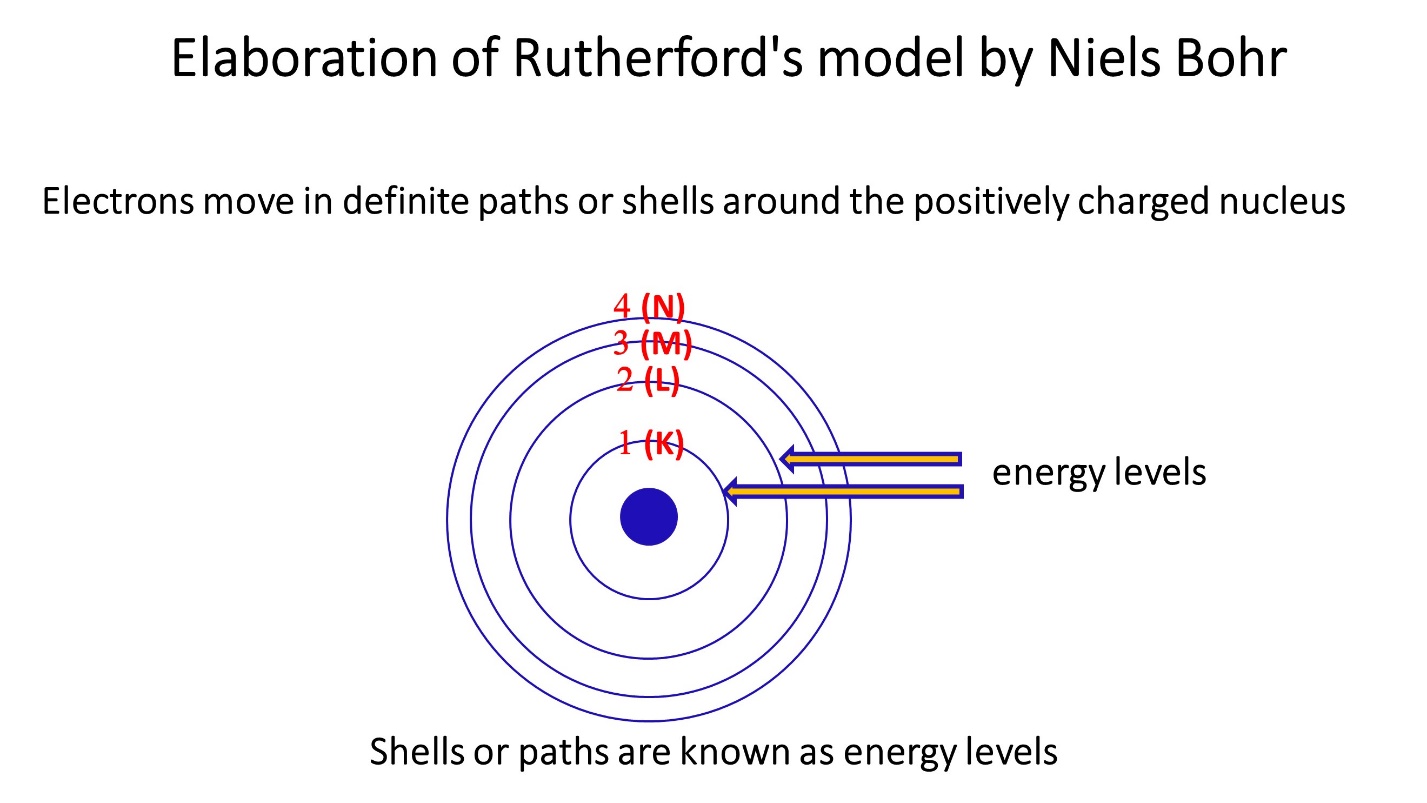
Elaboration of Rutherford’s model by Niels Bohr
Detailed history is in this article.
Around the positively charged nucleus, electrons flow in distinct routes or shells. These shells or paths are known as energy levels.
- Each of the energy levels have their own specific energy.
- When moving away from the nucleus this energy increases
- When moving away from the nucleus difference between the energy levels decreases
Maximum number of electrons in each energy level
Maximum 2 electrons in first energy level. Maximum 8 electrons in second energy level. Likewise maximum 18 electrons in third energy level and 32 electrons in fourth energy level.
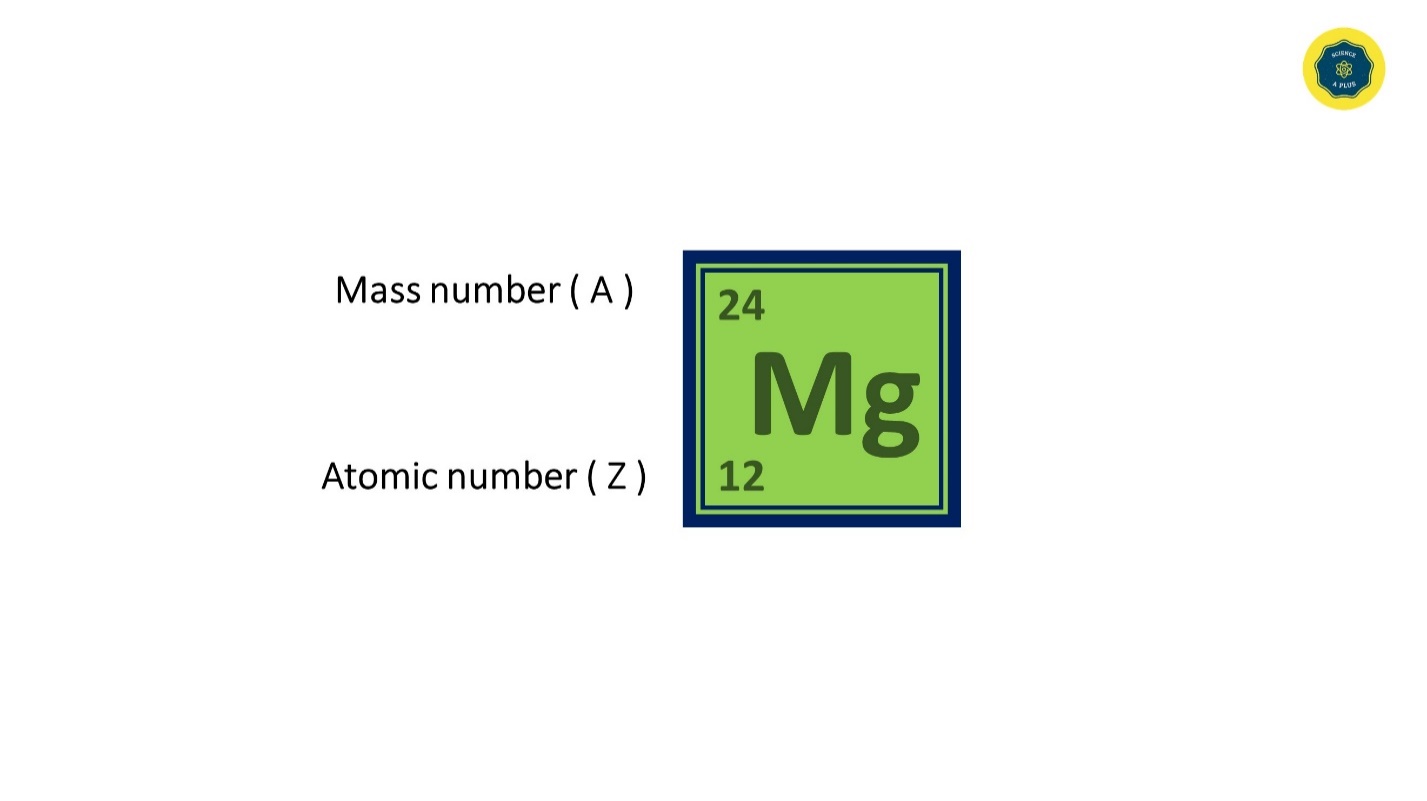
Mass Number and Atomic Number
Mass number is written in upper left corner of an element and Atomic number is written in lower left corner of an element.
Atomic number is equal to the number of electrons as well as the number of protons in an uncharged atom.
The mass number is equal to the sum of the number of protons and neutrons.
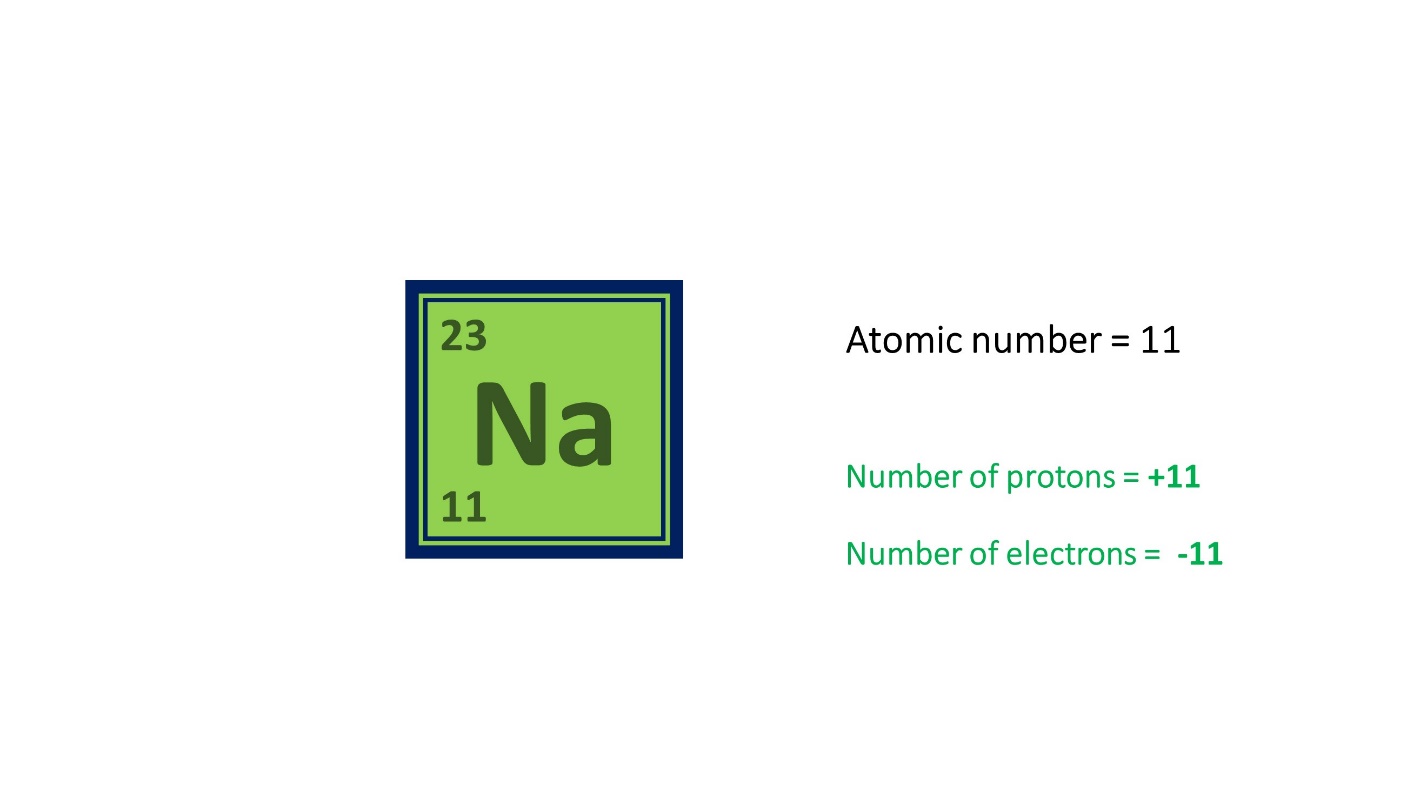
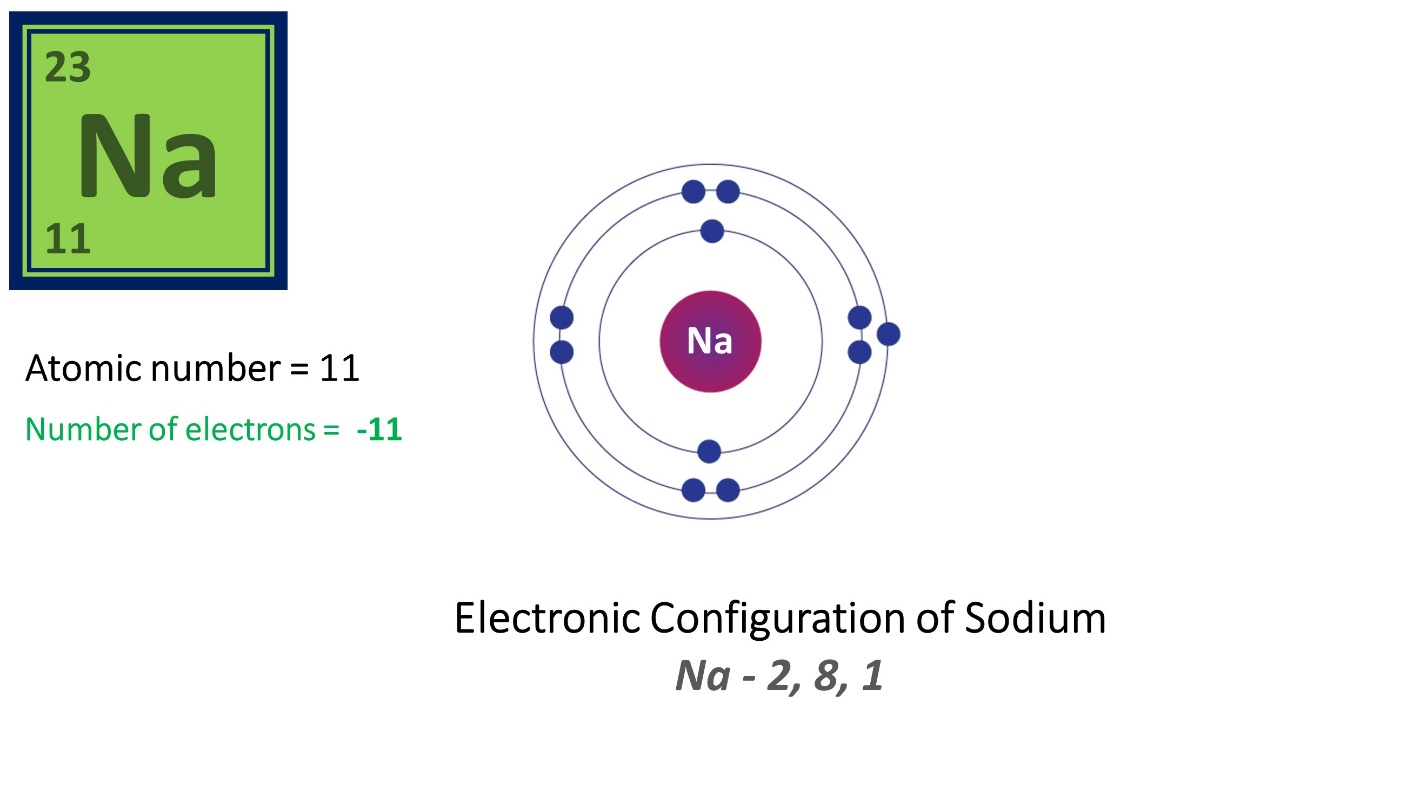
Here, Sodium (Na) has 11 protons and 11 electrons. Its atomic number is 11.
Since it has 11 protons and 12 neutrons its mass number is 23, which is the sum of 11 and 12.
What is electronic configuration of an atom?
Representing how electrons are filled in the respective energy levels from the one nearest to the nucleus of an atom to outwards.
Let’s see an example – Sodium
Electronic configuration of Sodium is 2,8,1
How to write the electronic configuration of an atom?
- Atomic number is the sum of electrons in an atom. For Sodium it is 11.
- Fill each energy level from inwards.
- First energy level gets 2 electrons, so 9 electrons are remaining.
- Let’s fill the second energy level. It can contain maximum 8 electrons.
- Keep 8 electrons in the second energy level and now we have 1 electron remaining.
- Put the remaining electron in the next (third) energy level.
- Now it is complete. Let’s look back at the energy levels.
- First energy level has 2 electrons and it is full. Second energy level is full with 8 electrons and the third energy level has only one electron.
- So, the electronic configuration is 2,8,1
Here some easy examples for writing electronic configuration.
- Hydrogen has one electron. So, its electronic configuration is simply 1.
- Helium has 2 electrons. Its electronic configuration is 2.
- Lithium has 3 electrons. 2 electrons fill the first energy level and the remaining electron stays in the next (second) energy level. The electronic configuration is 2,1.
- Beryllium with its 4 electrons has an electronic configuration of 2,2.
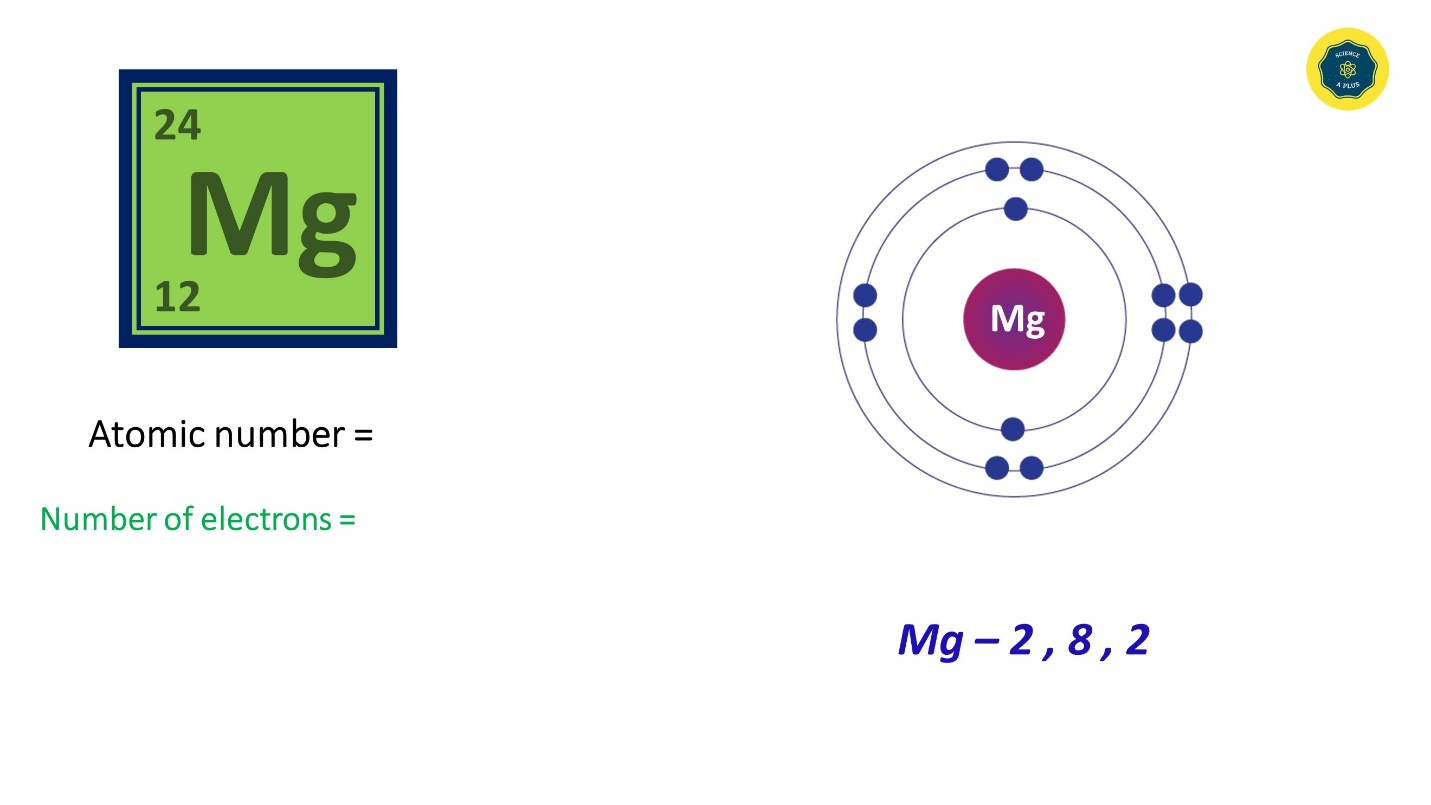
- Magnesium has 12 electrons and the electron configuration is 2,8,2. It has 12 electrons and the atomic number is also 12.
- The above two examples show that first energy level is filled with 2 electrons, second energy level is filled with 8 electrons and the third energy level is filled with 8 electrons.
- This results in pushing the remaining electrons to the fourth energy level.
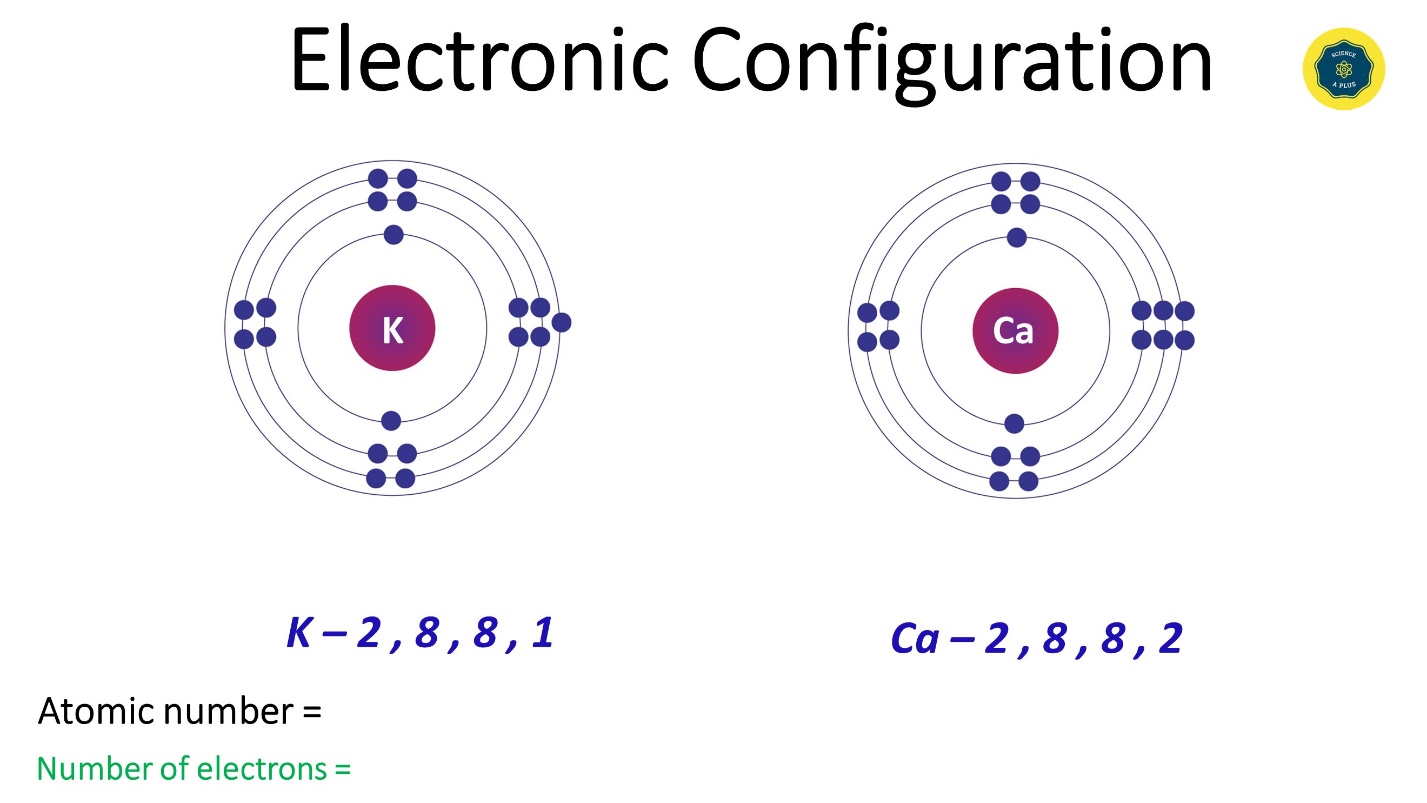
- Potassium (K) has an electron configuration as 2,8,8,1. Atomic number 19. No of electrons 19.
- Calcium (Ca) has its electronic configuration as 2,8,8,2. Atomic number 20. No of electrons 20.
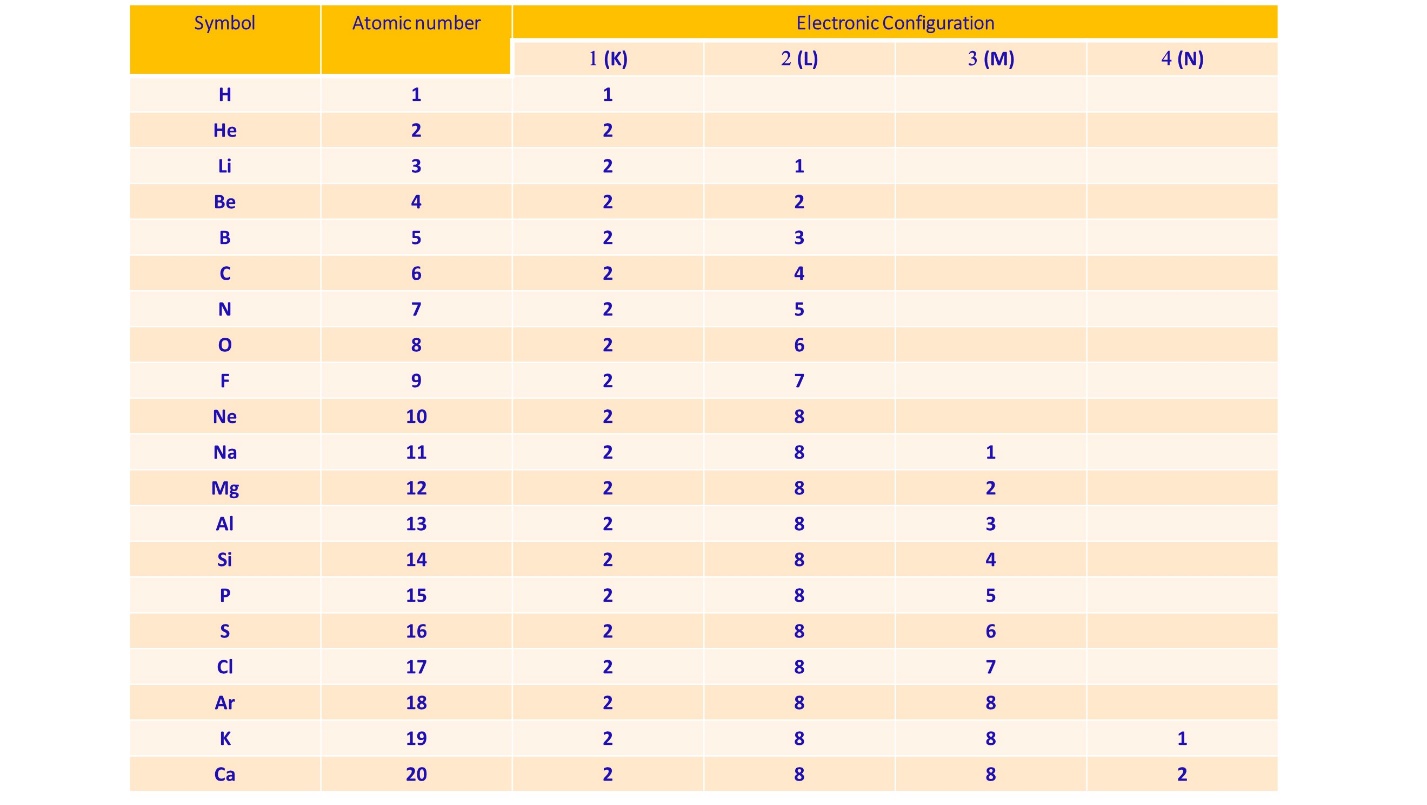
The below table shows the electronic configuration of the first twenty elements.