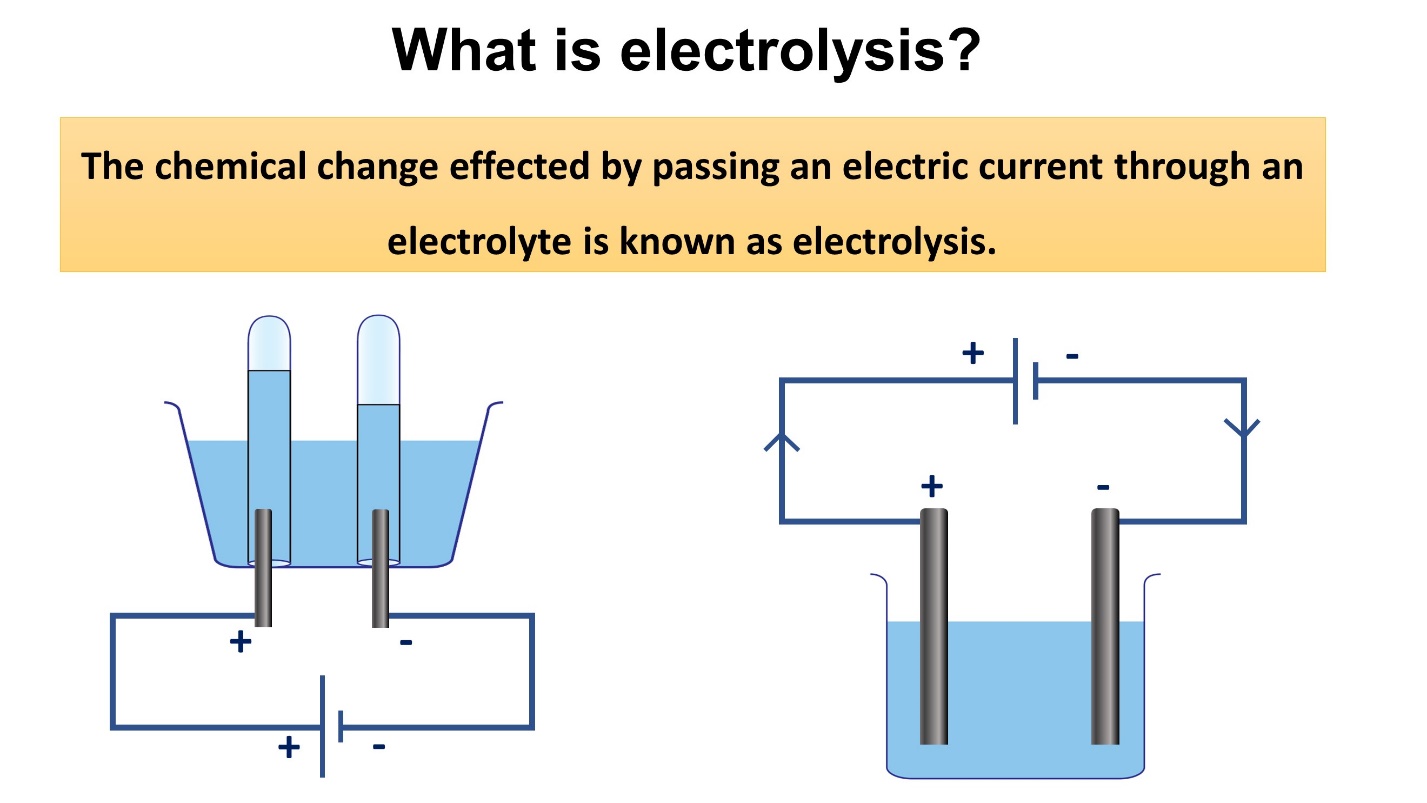
What is electrolysis?
The chemical change resulted by passing an electric current through an electrolyte is known as electrolysis.
During this process, the electrolyte (solution) is converted into simpler components.
Applications of electrolysis
- Extraction of certain metals (e.g. sodium, aluminum)
- Industrial manufacturing of sodium hydroxide (caustic soda)
- Electroplating
Electrolysis is commonly used in various industries. One popular application is Electroplating.
What is electroplating?
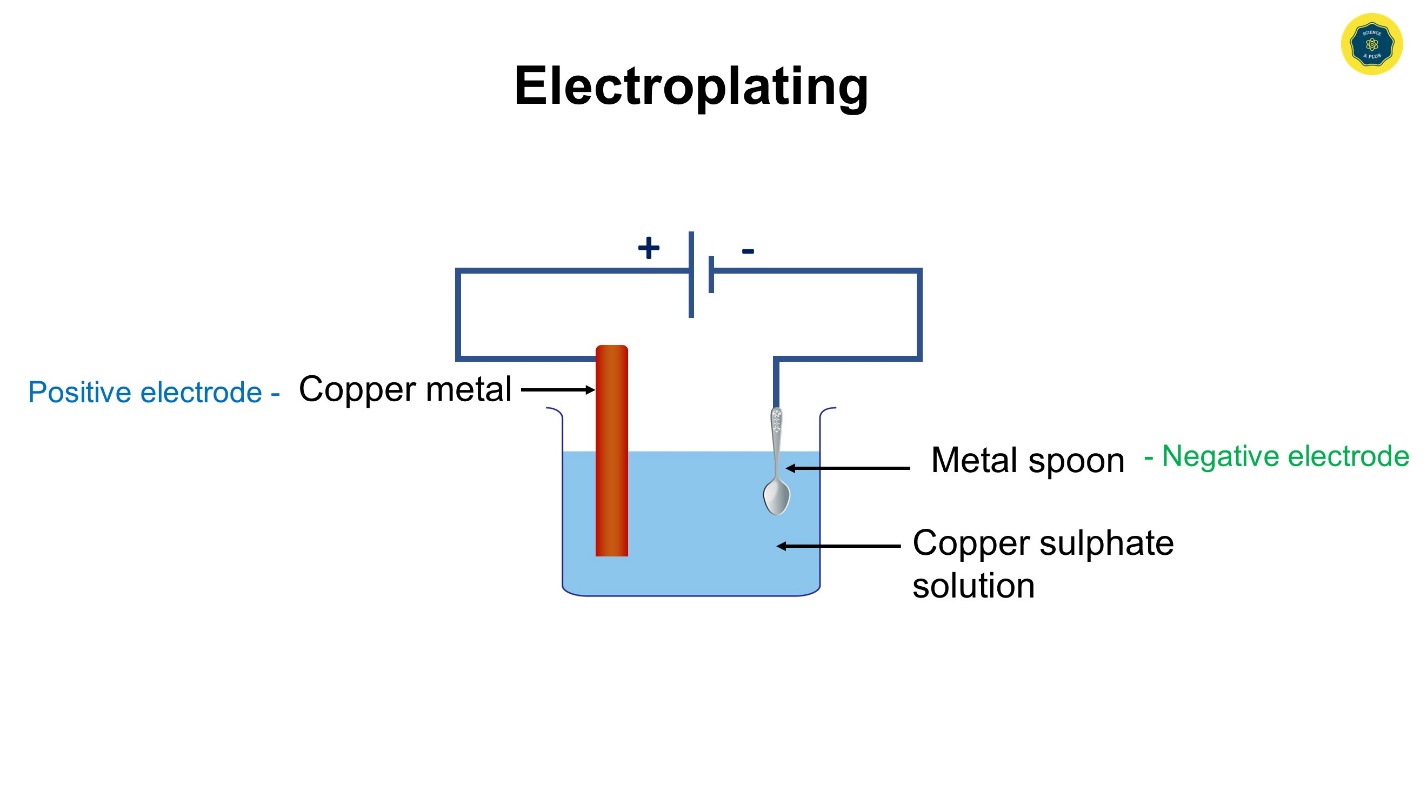
The plating of a certain metal on another surface with the use of electricity is referred to as, electroplating.
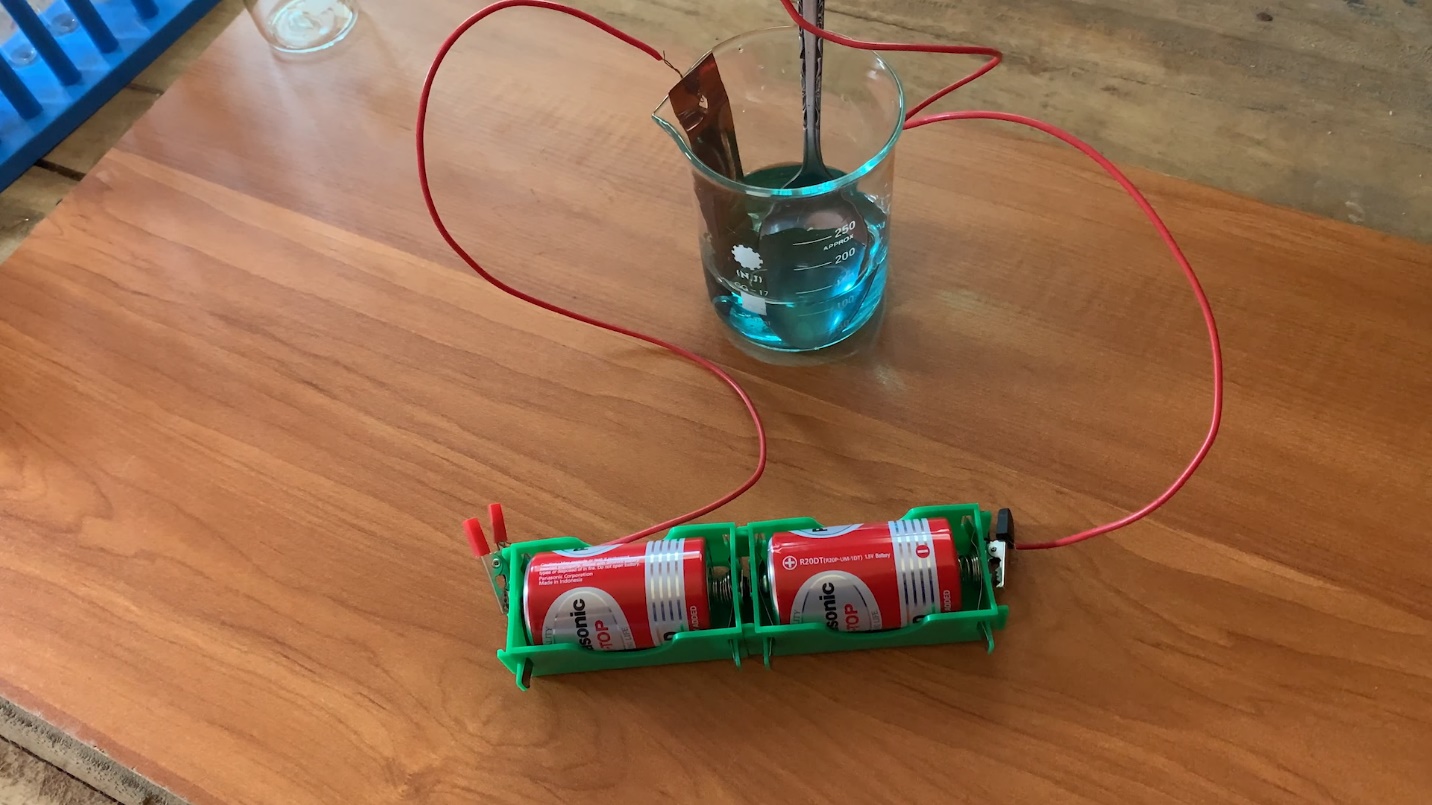
You need
Aqueous copper sulphate solution, a clean copper plate, a cleaned spoon, a beaker, connecting wires, and dry cells.
Method
- Add some copper sulphate solution to the beaker.
- Connect two connecting wires to the copper plate and iron spoon.
- Connect the free ends of the two connecting wires to the dry cell.
- Thereafter put the metal plate (copper) and the spoon into the beaker filled with copper sulphate solution, at once.
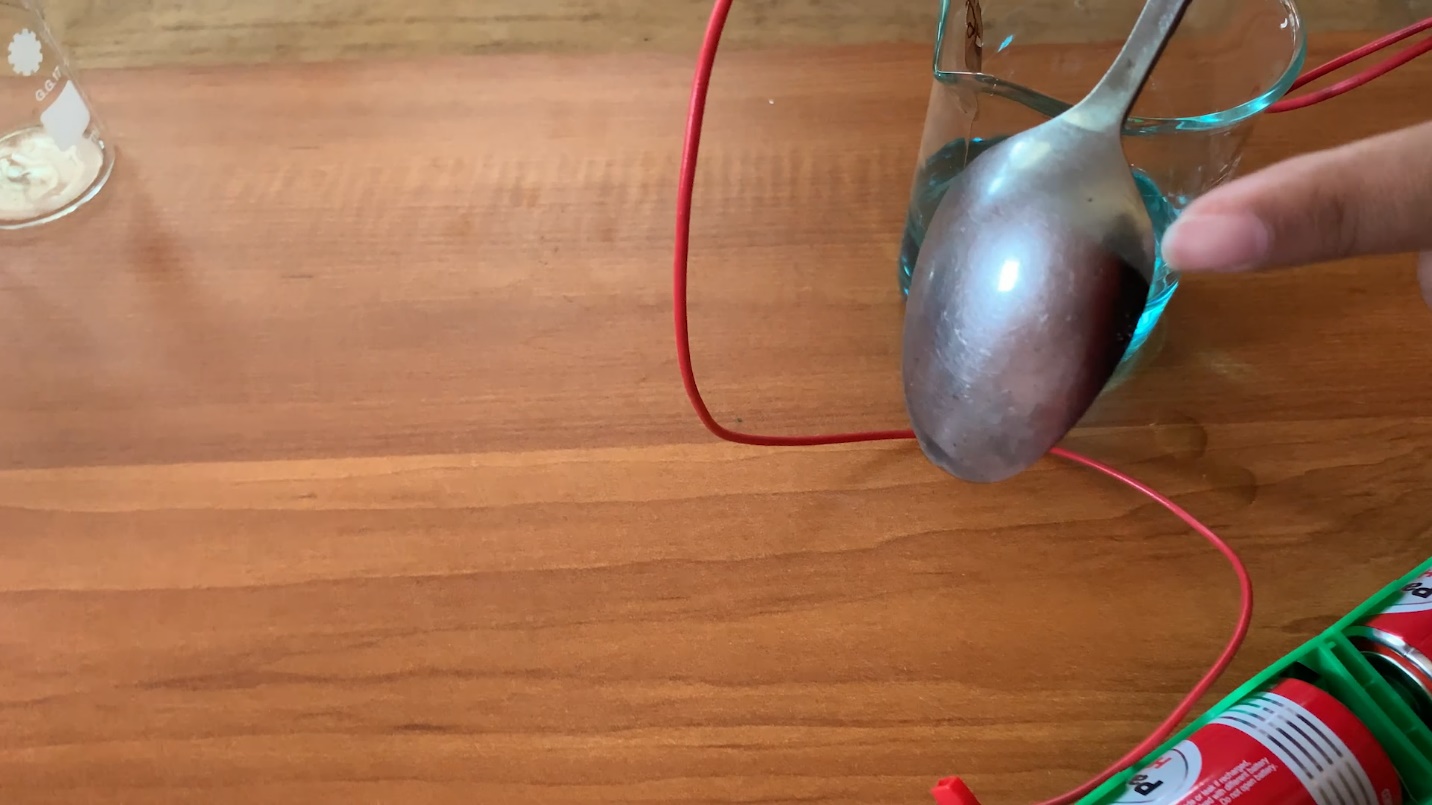
- Observe the spoon after a few minutes.
Observations
The part of the spoon dipped in copper sulphate solution, has turned into copper colour.
Conclusion
A thin layer of copper is deposited on the spoon due to the process called electrolplating.
Electroplating is an application of electrolysis.
Discussion
For electroplating, the metal that needs to be plated (copper) should be used as the positive electrode and, the object that is plated (spoon), should be used as the negative electrode.
The electrolyte needs to be a solution of a salt of the metal that should be plated. (copper sulphate is a salt of copper metal used here)
Characteristics of high-quality electroplating;
- The coating should firmly adhere to the surface subjected to the plating.
- The coating should be of even thickness.
- The coating should be shiny.
How to do a high-quality plating?
High-quality plating can be done when the electrochemical change takes place very slowly. The speed of this process can be reduced by passing a very low current through the solution.
The electrolyte (salt solution) used for the process should be dilute.
In industrial chemistry, high-quality electroplating is achieved by controlling the above conditions precisely.
Applications of electroplating
- Plating of metals like nickel and chromium onto iron.
- Preventing rusting of the parts of motor vehicles by coating them with a thin metallic layer.
- Copper jewelry is coated with gold metal.