You will need
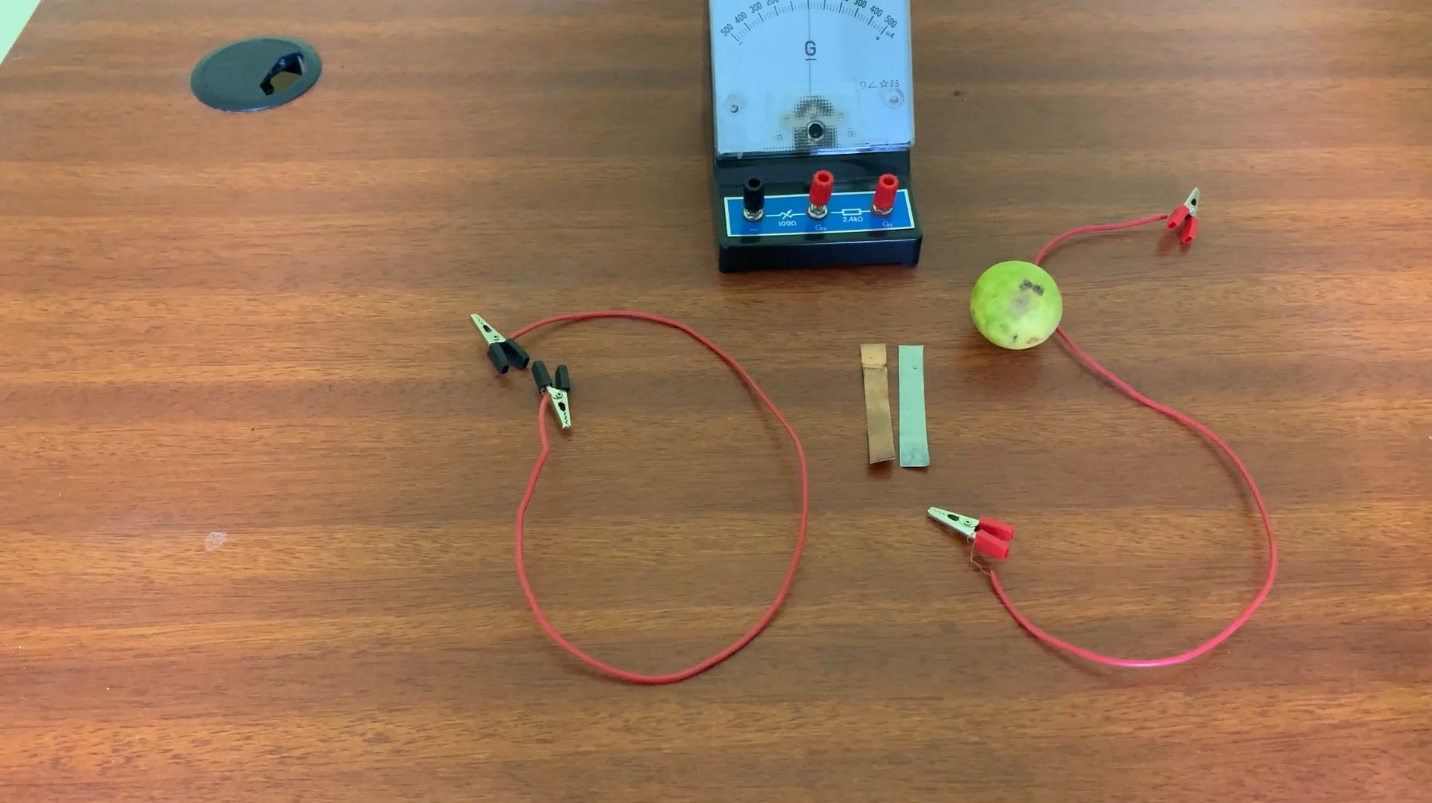
A lime fruit, a plate of copper, a plate of zinc, connecting wires, and a galvanometer.
Method
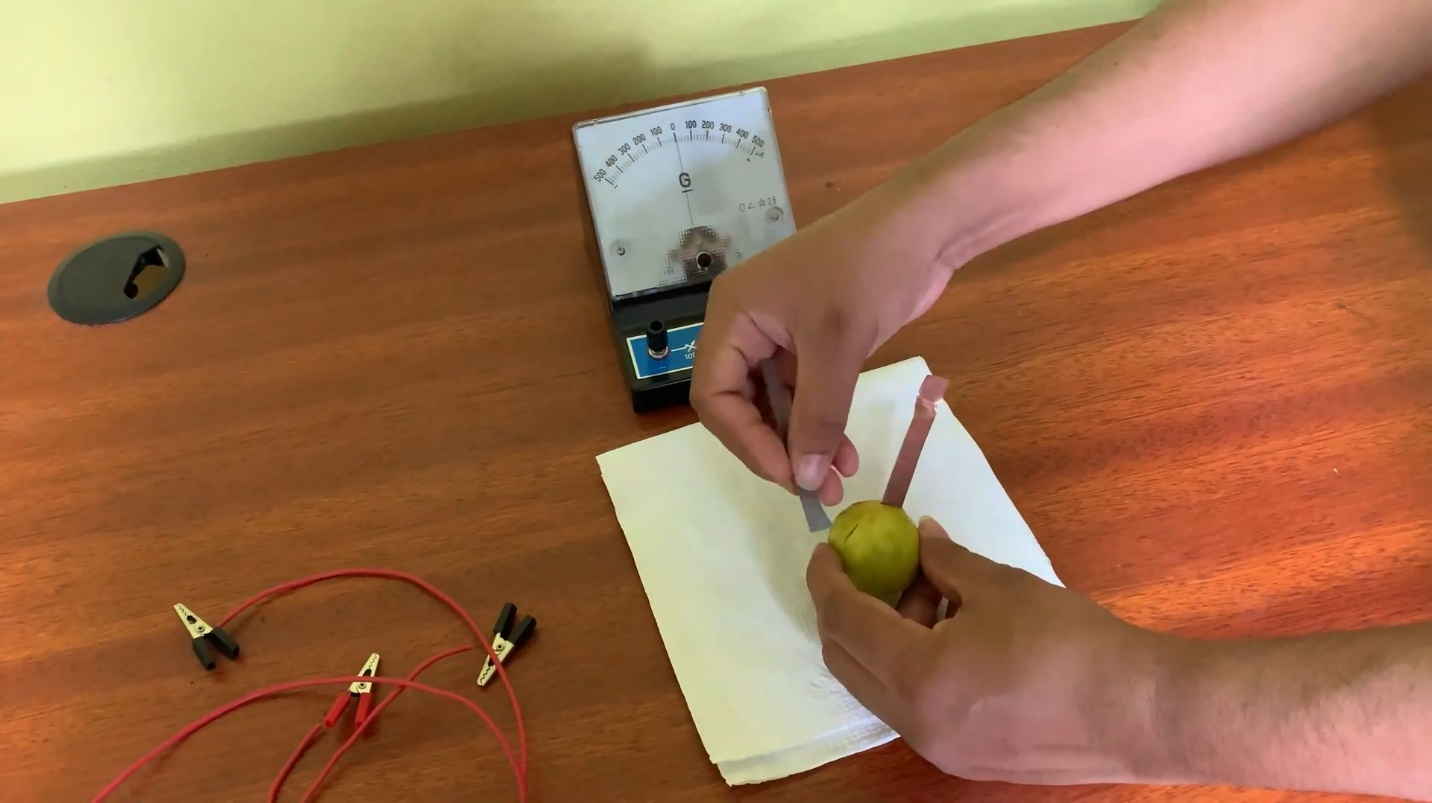
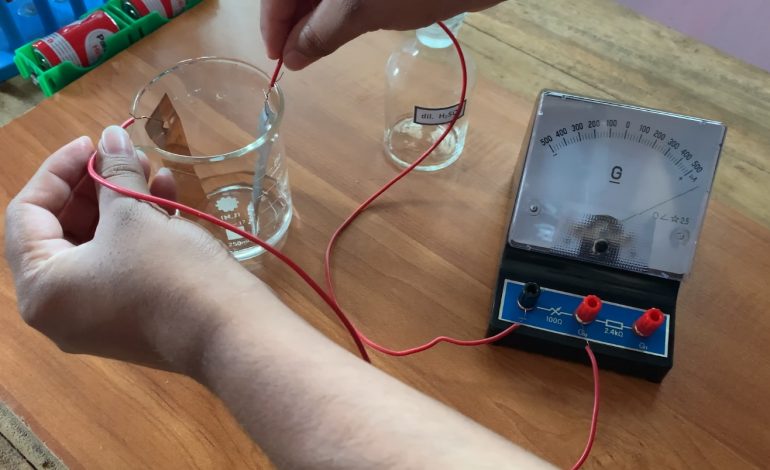
- lime fruit should be thoroughly pressed to liquidize its juice.
- Insert the parts of copper sheet as well as the zinc sheet into lime fruit.
- Connect a wire to each of the metal sheets.
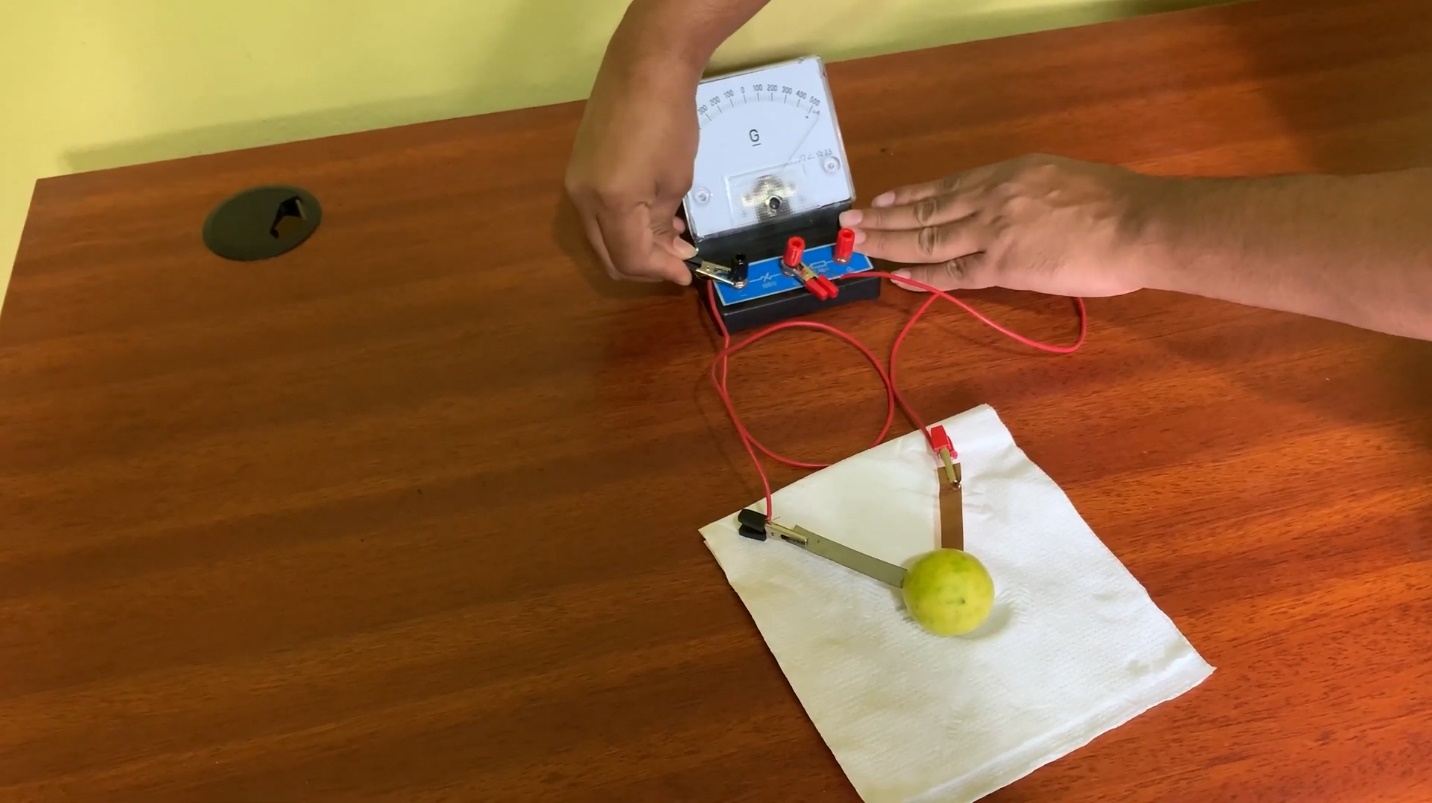
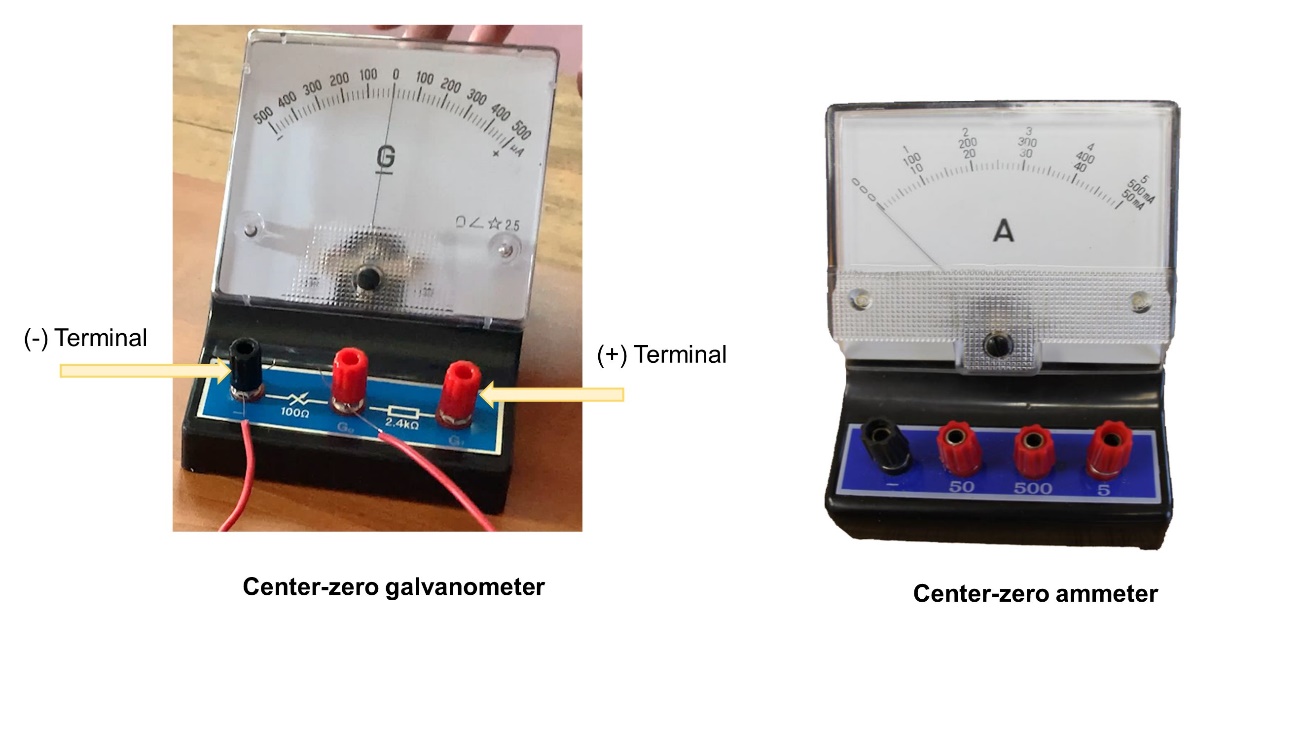
- Connect those wire ends to the galvanometer.
- Copper sheet and zinc sheet should be taken as positive and negative terminals, respectively.
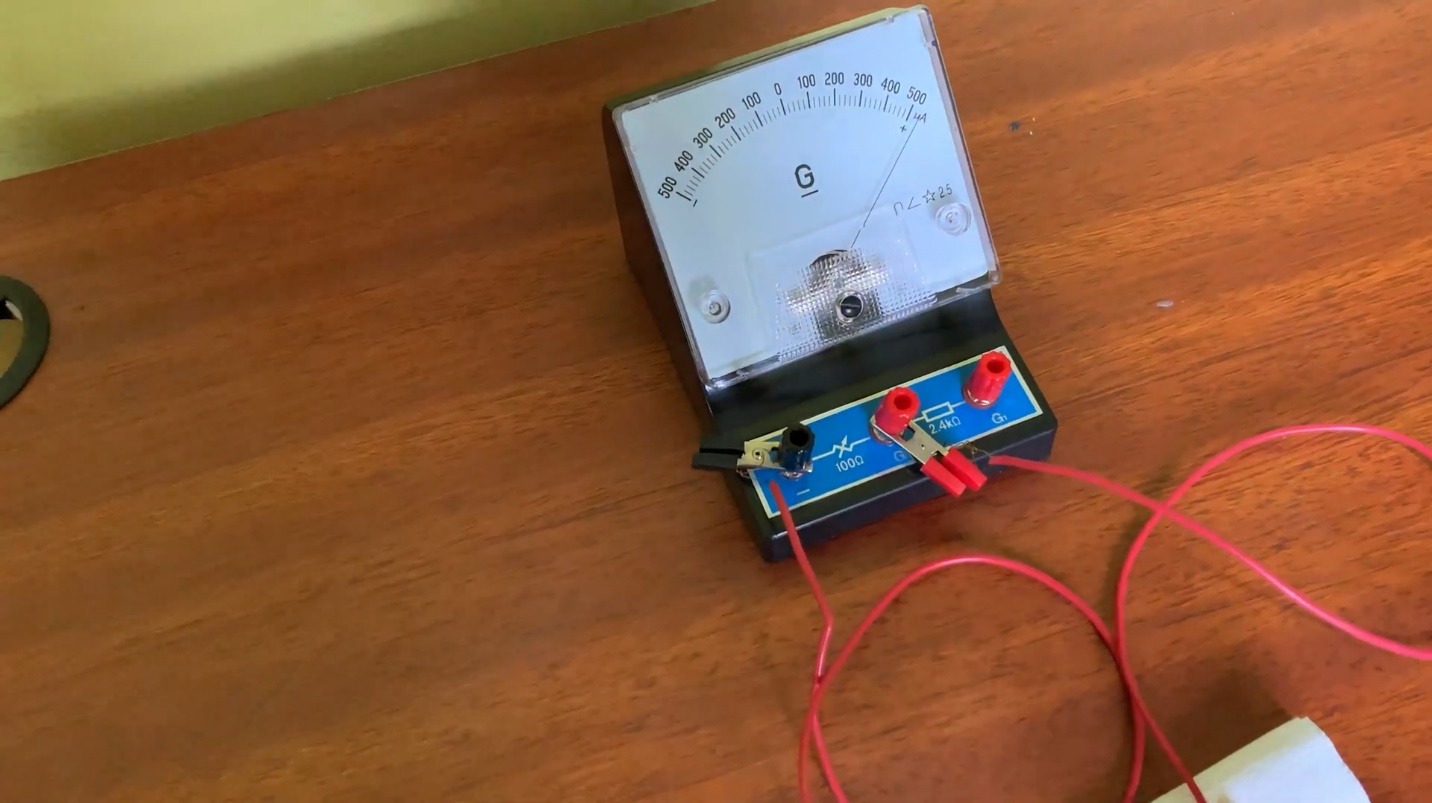
- Observe the indicator of the galvanometer.
Observations
- The indicator of the galvanometer deflected
Consider
Consider, that the positive and negative terminals of the galvanometer should be connected to the copper rod and zinc rod respectively.
Conclusion
The needle of the galvanometer starts to deflect. It indicates that a small current is produced, in the lime fruit, and it flows through the circuit.
Electricity is generated by a chemical process, in some sources of electricity like dry cells, simple cells, and car batteries.
A galvanometer is used to identify a small current that flows through a circuit.