Titanium is a chemical element and its symbol is Ti and the atomic number is 22. It is a lustrous transition metal with a silver color, and it is known for its excellent strength-to-weight ratio, corrosion resistance, and biocompatibility. These properties make it an attractive material for a wide range of applications, including aerospace, automotive, medical, and sports industries.
Discovery and Occurrence:
Titanium was discovered by the British chemist William Gregor in 1791, who named it menachanite after the place where he found it, Menaccan in Cornwall, England. However, it was not until 1795 that the German chemist Martin Heinrich Klaproth identified it as a new element and named it titanium after the Titans of Greek mythology.
Titanium is the ninth most abundant element in the Earth’s crust and is found in minerals such as ilmenite, rutile, and anatase. The main sources of titanium are Australia, South Africa, Canada, and the United States.
Properties and Uses:
One of the most remarkable properties of titanium is its strength-to-weight ratio, which is the highest of all metallic elements. This property makes it an ideal material for use in the aerospace and automotive industries, where it is used in the manufacture of aircraft, spacecraft, and high-performance cars.
Another important property of titanium is its excellent corrosion resistance, which is due to the formation of a thin, protective oxide layer on its surface when exposed to air or water. This property makes it ideal for use in marine and offshore applications, such as shipbuilding, oil rigs, and desalination plants.
In addition to its mechanical and corrosion-resistant properties, titanium is also biocompatible, which means that it is not harmful to living tissue and can be used in medical implants, such as hip and knee replacements, dental implants, and pacemakers.
Titanium is also used in sports equipment, such as golf clubs, bicycle frames, and tennis rackets, due to its lightweight and strong properties.
Production and Processing:
The production of titanium involves several steps, including mining, processing, and refining. The most common method of production is the Kroll process, which involves the reduction of titanium tetrachloride with magnesium to produce pure titanium.
Once produced, titanium can be further processed through several methods, including forging, extrusion, and welding. These methods are used to shape the material into various forms, such as sheets, rods, and tubes, which can then be used in a wide range of applications.
Reactions of titanium
Titanium, being a transition metal, can participate in a variety of chemical reactions. Here are some common reactions of titanium, along with the corresponding chemical equations:
Combustion Reaction:
Titanium can undergo combustion in the presence of oxygen to produce titanium dioxide.
2Ti(s) + O2(g) → 2TiO2(s)
Reduction Reactions:
Titanium can be reduced by a variety of reducing agents, such as magnesium, sodium, or aluminum. These reactions produce titanium in its elemental form.
TiCl4(l) + 4Mg(s) → Ti(s) + 4MgCl2(l)
TiCl4(l) + 4Na(s) → Ti(s) + 4NaCl(s)
Acid-Base Reactions:
Titanium can react with acids or bases to produce various compounds. For example, reaction with hydrochloric acid produces titanium chloride.
Ti(s) + 2HCl(aq) → TiCl2(aq) + H2(g)
Reaction with sodium hydroxide
Produces sodium titanate.
TiO2(s) + 2NaOH(aq) → Na2TiO3(aq) + H2O(l)
Oxidation-Reduction Reactions:
Titanium can participate in redox reactions, such as the reaction with sulfuric acid and potassium permanganate to produce titanium dioxide.
Ti(s) + 4H2SO4(aq) → Ti(SO4)2(aq) + 4H2O(l) + SO2(g)
Ti(SO4)2(aq) + 2KMnO4(aq) → TiO2(s) + Mn2O3(s) + K2SO4(aq) + H2SO4(aq)
Coordination Complex Formation:
Titanium can form coordination complexes with ligands, such as water or ammonia. These reactions are important in the field of organometallic chemistry.
TiCl4(l) + 2H2O(l) → Ti(OH)2Cl2(s) + 2HCl(aq)
Titanium dioxide
Titanium dioxide (TiO2) is a white, odorless, and naturally occurring oxide of titanium. It is a versatile material with a wide range of applications due to its unique physical and chemical properties.
Production:
Titanium dioxide is produced by two main methods:
The sulfate process and the chloride process. In the sulfate process, ilmenite ore is reacted with sulfuric acid to produce a titanium sulfate solution, which is then hydrolyzed to form titanium dioxide.
The chloride process involves the reaction of titanium-containing ores with chlorine gas to produce titanium tetrachloride, which is then reacted with oxygen to form titanium dioxide.
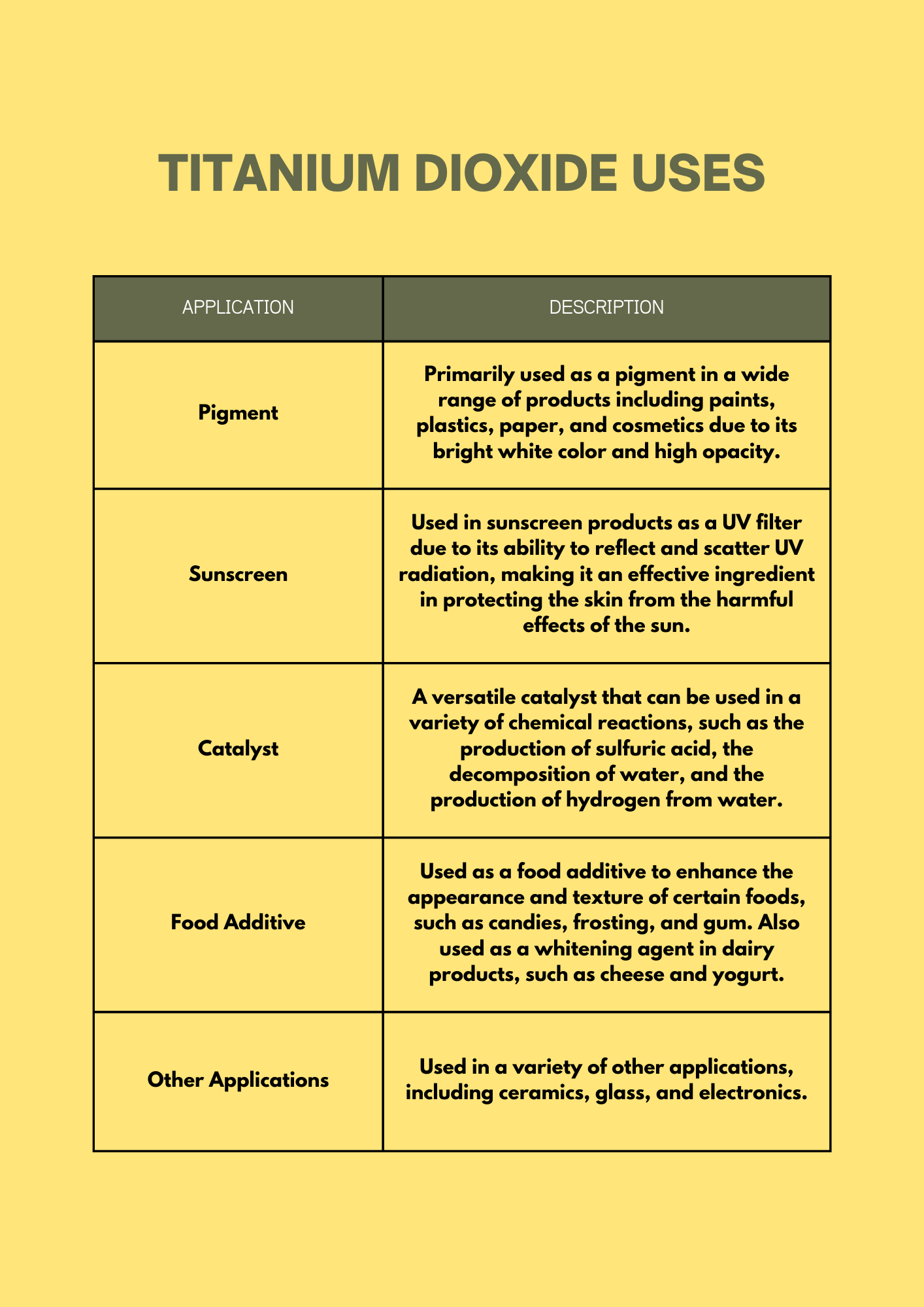
Applications:
Safety:
Titanium dioxide is generally considered safe for use in consumer products. However, there is some concern about its potential health effects when inhaled. Some regulatory agencies, such as the European Union, have placed restrictions on the use of titanium dioxide in certain applications, such as in food products.