Compound separation in mixtures by evaporation method
What is vaporization?
Vaporization, also known as evaporation, is the process by which a liquid substance changes into a gaseous state. This occurs when the molecules of the liquid gain enough energy to break free of the forces that bind them together and escape into the air as a gas or vapor.
When the temperature of a liquid is raised to its boiling point, which is the temperature at which the vapor pressure of the liquid equals the atmospheric pressure, vaporization occurs. The liquid will begin to boil and turn into a gas at this point. However, at temperatures below the boiling point, some molecules in the liquid can gain enough energy to overcome the intermolecular forces that hold them together and escape into the air as a gas.
How to separate components of a mixture by Vaporization/Evaporation?
When heat is applied to a mixture, the process of vaporization causes the unnecessary components to evaporate, and it then separates the essential component from the mixture.
How to obtain pure gold by evaporation?
A special solution known as an amalgam is formed when metals are dissolved in mercury.
When impure gold is dissolved in mercury, a pure gold solution is obtained. This is referred to as the gold amalgam. When gold amalgam is heated, the mercury evaporates, leaving only pure gold.
Extraction of salt by evaporation
Perhaps you’ve watched salt being extracted from the ocean. The water evaporates because of the sun’s heat. The process of evaporation leads to the precipitation of the dissolved salts.
Compound separation in mixtures by evaporation by Filtration
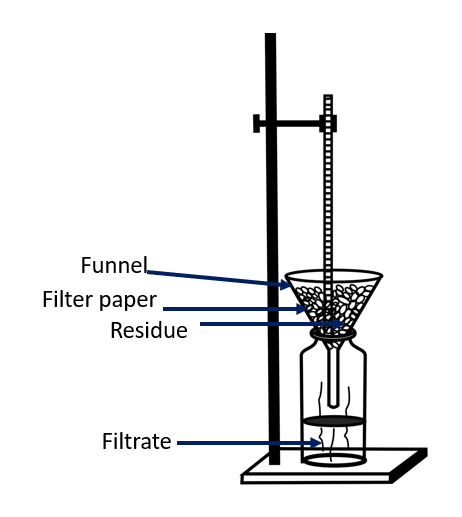
Filtration can be used to separate components from a mixture that remain suspended in a liquid but do not dissolve in the solution. To filter a mixture, a filter is required.
A milk strainer is one such device. Another type of filter is the filter paper used in laboratories. Sand filters are used in water purification plants.
Small holes are present in a filter. Smaller particles can pass through the holes. Particles larger than those holes, however, cannot pass through them. This is the idea behind filtration. The substance left in the filter after filtration is known as the residue, and the solution that is filtered is known as the filtrate.
Filtration apparatus
Compound separation in mixtures by evaporation by Crystallization
What is Crystallization?
Crystallization is the formation of a solid crystal from a solution, melt, or gas. It’s a popular separation technique for purifying substances or obtaining solid forms of materials like pharmaceuticals, chemicals, and minerals.
The solute (the substance to be crystallized) is dissolved in a solvent during the crystallization process (the liquid in which it is dissolved). The solution is then cooled or allowed to slowly evaporate, causing the solute to precipitate and form crystals. Because the solute molecules become more ordered and arrange themselves in a regular, repeating pattern, crystal formation occurs.
When a solute that can solidify is present in a solution, crystallization is the method of separating solid substances by concentration.
Consider situations in which a solid dissolve in a solvent to form a homogeneous mixture. A maximum concentration of a substance that remains dissolved in a solution exists at a specific temperature. This substance is said to be saturated in such solutions.
When this saturated solution is vaporized, the concentration of that substance in the solution grows even more. When the solute concentration exceeds the maximum possible concentration in the solution, the solute separates and crystals form.
Applications of Crystallization
Crystallization is used in the sugar manufacturing industry. Sugarcane stems are crushed and squeezed, and the juice is purified. Vaporization increases its concentration. The sugar crystals then separate from the juicy solution.
Another industry that uses crystallization is the production of salt from sea water. Several salts that are dissolved in sea water crystallize during the salt production process in salterns.
Compound separation in mixtures by evaporation by Recrystallization
Recrystallization
Recrystallization is a technique for separating pure substances from solid, crystalline substances that contain impurities. Recrystallization is the process of dissolving a solid, crystalline substance and re-forming it into crystals. Recrystallization can produce high-quality crystals free of impurities.
Recrystallization process
The impure solid is dissolved in the hot solvent until it becomes saturated during recrystallization. Following that, while the solution is still hot, it is filtered to separate the impurities in the impure solid. Cooling the filtrate yields pure crystals of the solid. Because the cold solution is saturated with the solute but the hot solution is not, crystallization occurs. Because the solution is not saturated in them, the soluble components present as impurities in minor amounts do not crystallize.
Solvent extraction
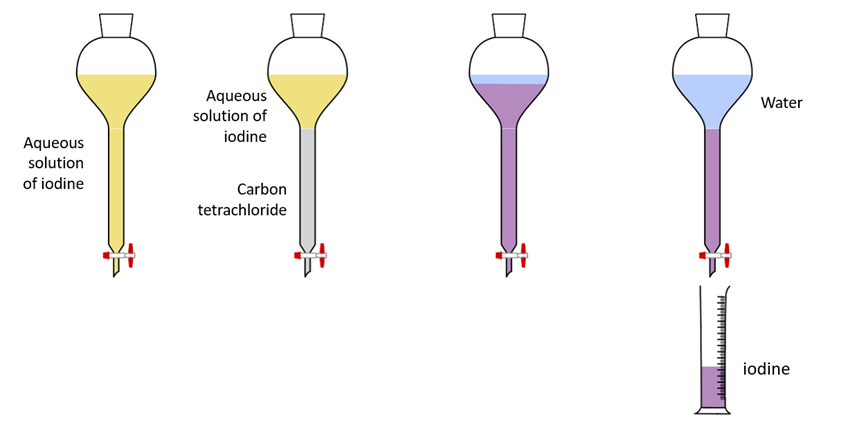
Some solutes are soluble in large amounts in one solvent but dissolve in minute amounts in another. When solid iodine is added to water, a very small amount dissolves, resulting in a light-colored solution. However, iodine dissolves more readily in solvents such as carbon tetrachloride and cyclohexane.
Separation of iodine by solvent extraction
When carbon tetrachloride is added to an aqueous iodine solution, it does not mix, and the layers separate. After a while, the carbon tetrachloride layer turns violet, while the aqueous layer becomes pale. Iodine has been extracted from the aqueous layer and transferred to the carbon tetrachloride layer, where it is more soluble. The ability of a small volume of carbon tetrachloride to extract iodine from a large volume of aqueous solution is the specialty here.
Iodine can then be recovered by layer separation and evaporation of carbon tetrachloride.
As a result, solvent extraction is the process of drawing a substance from a less soluble solvent into a more soluble solvent, where the two solvents are immiscible and in contact with each other.
Solvent extraction apparatus – Separation of iodine
Other applications of solvent extraction
The medicinal components of certain plants are only present in extremely minute quantities. The use of a solvent such as ethanol allows for the preparation of medicinal solutions of a higher concentration. The manufacture of medicinal extracts and tinctures frequently involves the use of solvent extraction.
Compound separation in mixtures by distillation
Simple distillation
Simple distillation is used to separate components in a mixture that includes a volatile component as well as non-volatile components. During distillation, only the volatile components are vaporized. The remaining components are still in the solution.
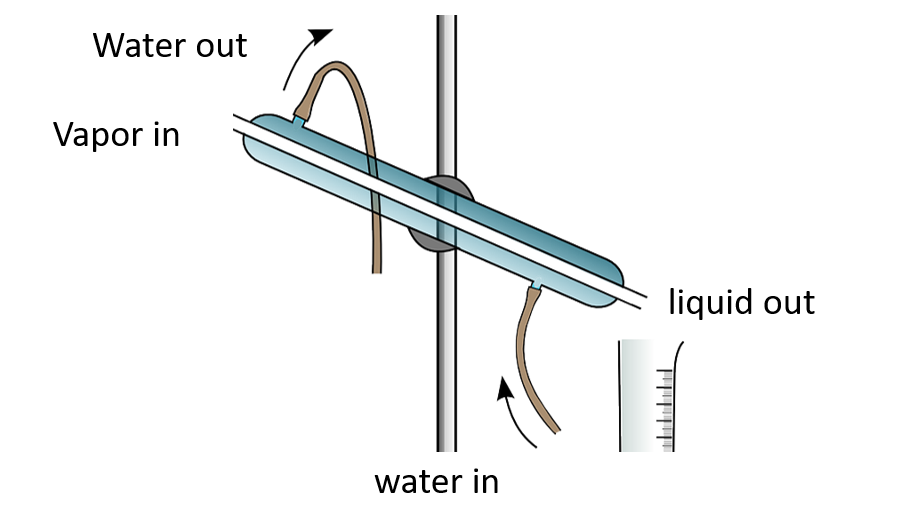
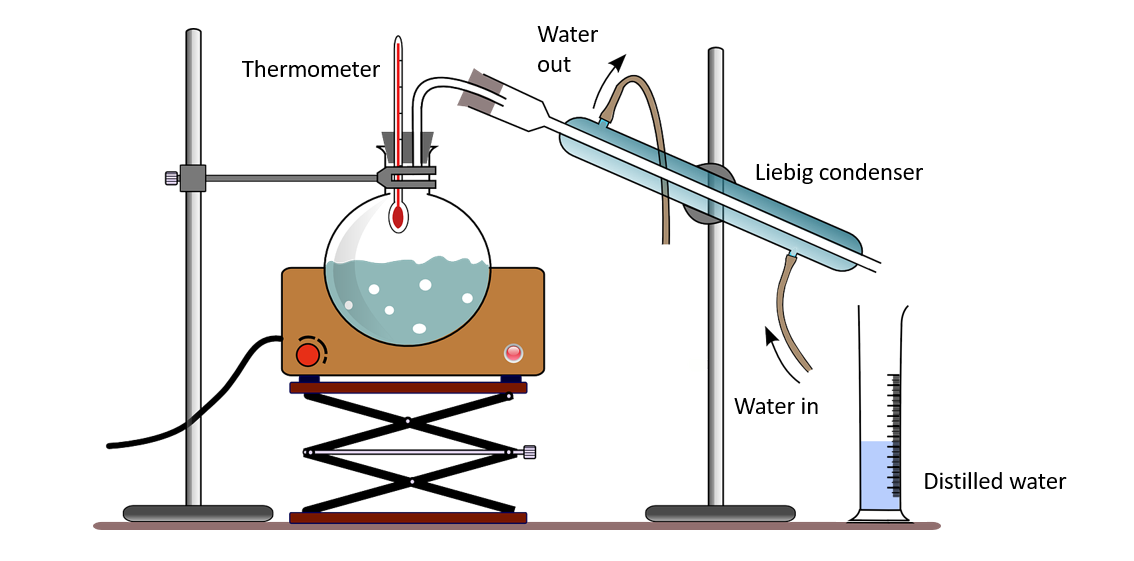
This method is used by some countries to obtain potable water from sea water. It contains salts in addition to water. Salts have higher boiling points than water. As a result, when sea water is heated and vaporized, only water evaporates. Deposited salts can be seen. Special temperature control is not required for this type of distillation. As a result, this is referred to as simple distillation. Simple equipment, such as the Liebig condenser, is adequate for this.
Simple distillation apparatus
Simple distillation apparatus – Named diagram
Fractional distillation
Fractional distillation is the separation of several components by distillation under controlled cooling conditions.
If the solution or mixture to be separated contains several volatile components, simple distillation or the simple distillation apparatus cannot be used to separate them. It must be carried out under controlled conditions, and a fractionating column should be used. If two liquids are to be separated by fractional distillation, their boiling points should be significantly different. That means their volatilities must be very different. The vapor in this case contains a higher proportion of the more volatile component and a lower proportion of the less volatile component.
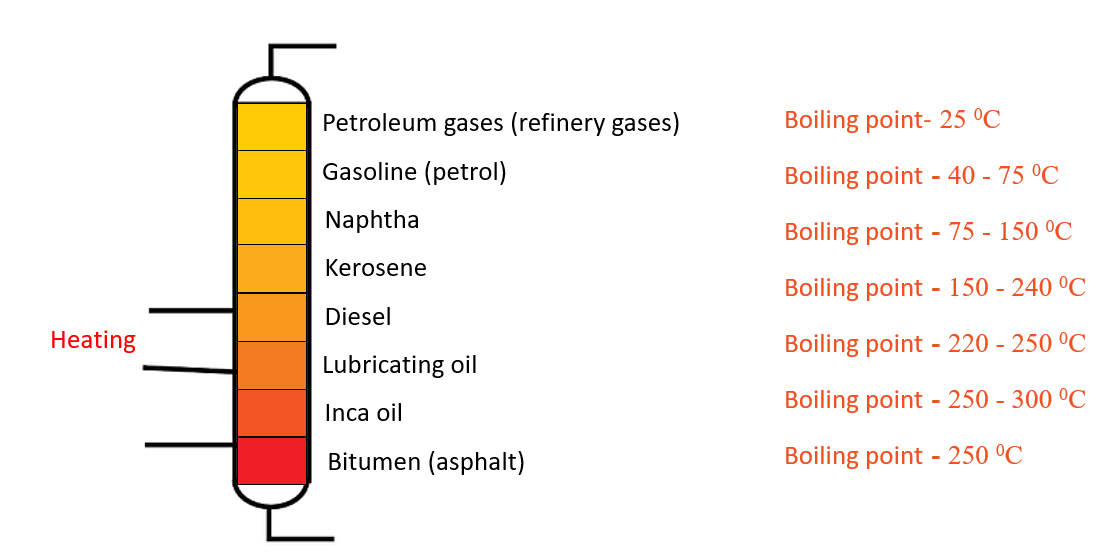
Fractional distillation apparatus – Separate components in crude oil
Separate components in crude oil
Crude oil is a complex mixture of hydrocarbons. To control the cooling conditions during the refining of crude oil, a fractionating tower is used. The temperature in this tower is appropriately controlled at various levels, and the components are withdrawn separately at their respective positions. Lower boiling point components are separated from the tower’s upper levels. At the bottom of the tower, components with high boiling points (bitumen) are deposited.
Steam distillation
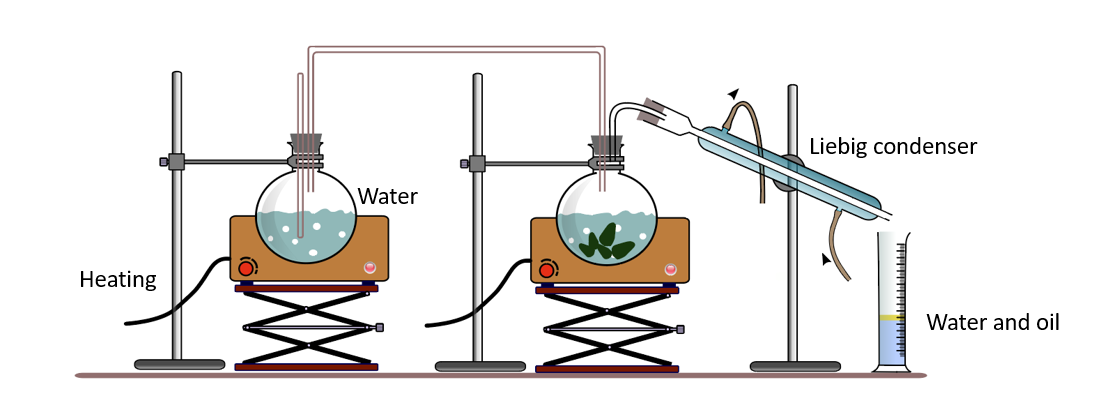
We already know that certain plant parts contain volatile components. Some examples include cinnamon, clove, nutmeg, and cardamom. It is difficult to raise the temperature uniformly to these compounds’ boiling points. Furthermore, at temperatures close to the boiling point, these compounds may be destroyed by decomposition or converted into other compounds. As a result, steam provides heat to the mixture.
When water soluble compounds are mixed with water, their boiling points rise above water’s boiling point. When compounds that do not mix well with water are mixed with water, the boiling point of the mixture falls below the boiling point of water.
The majority of essential oils are immiscible with water and have higher boiling points than water. They can be found in living cells, mixed with water. The extraction of essential oils in the laboratory can be demonstrated using an apparatus such as the one shown below.
When these mixtures are heated with steam, both water and essential oil are liberated as a mixture of vapors at temperatures below the boiling point of water (100 0C). Because water and essential oils are immiscible, when the distillate (vapor) is cooled, it separates into two layers. As a result, they are easily separated as pure substances.
Steam distillation apparatus
Compound separation in mixtures chromatography
What is chromatography
Chromatography is a technique for separating and identifying non-volatile components in a mixture (solid or liquid). There are numerous types of chromatography. Paper chromatography refers to the method of using paper (cellulose).
When several components are mixed together, the chromatographic technique can be used to separate and identify them. Chromatography is used to determine whether or not poisonous chemicals have been mixed with water. It is also used to determine whether harmful substances are present in food. Chromatographic analysis can also be used to identify active chemical compounds in plants.
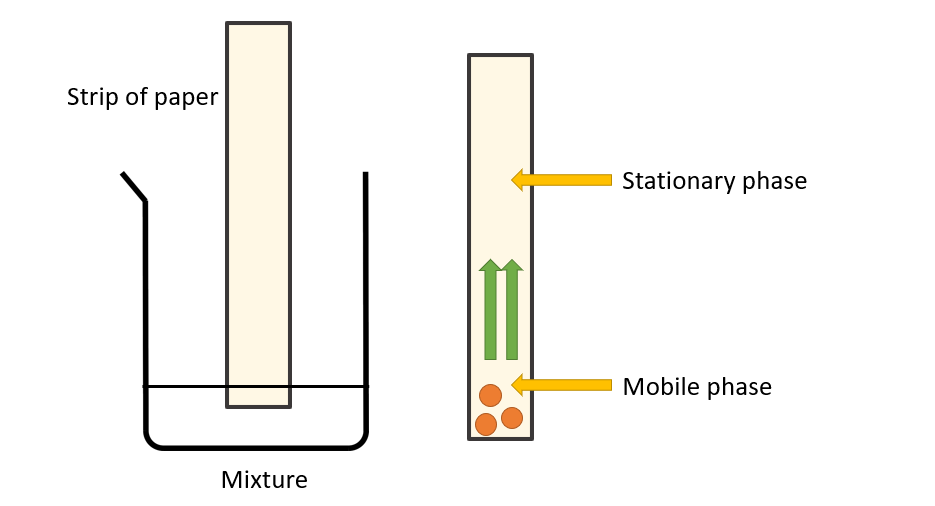
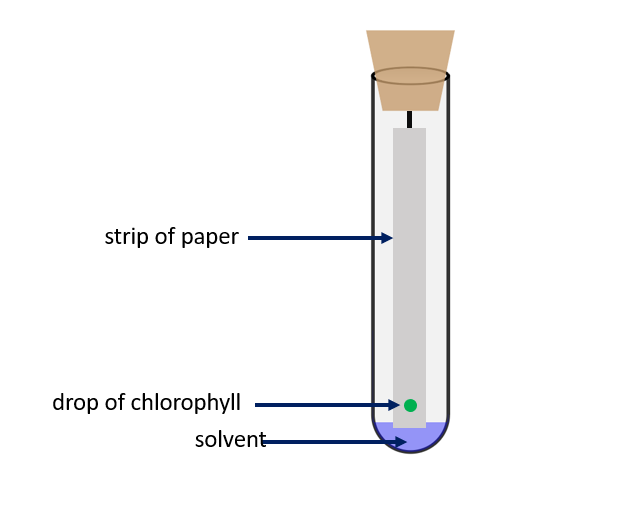
How to prepare chromatography setup?
Fill a petri dish halfway with water and dip one end of a dry strip of filter paper in it. The strip of paper is being soaked up by a stream of water particles from bottom to top. Even when water is replaced by compounds like acetone, ether, and ethyl alcohol, a flow of liquid can be seen rising from the bottom to the top. The stationary phase is the strip of paper, and the mobile phase is the solvent that is soaked into it. When a small amount of the mixture’s components that need to be separated is placed on this paper, the components dissolve in the solvent and move up with the solvent front. This upward movement is determined by the forces of attraction of the mixture’s components to the stationary phase. For example, if one of the components in the mixture is strongly attracted to the stationary phase (the paper), the rate of upward movement slows. If another component in the mixture is less attracted to the mixture, it rises faster through the stationary phase. The components of the mixture become separated from one another due to the difference in their rates of movement.