Weak Acid Strong Base Titration
In a weak acid-strong base titration, a weak acid is titrated with a strong base to determine its concentration. The aim of the experiment is to add the base to the acid in small amounts until the solution becomes neutral. The endpoint is reached when the pH of the solution is equal to 7. The pH of the solution is measured throughout the titration process using a pH meter. As the base is added, the pH of the solution increases gradually. However, when the equivalence point is reached, the pH of the solution jumps to a higher value, indicating that all the acid has been neutralized.
During the titration process, the concentration of the weak acid is determined by using the known concentration of the base and the volume of base required to reach the equivalence point.
Titration definition chemistry
Titration is a common laboratory technique used in chemistry to determine the concentration of an unknown substance in a sample. The process involves the gradual addition of a standardized solution of a known concentration (the titrant) to the solution containing the unknown substance until the reaction between the two is complete. The point at which the reaction is complete is known as the equivalence point, and it can be determined by monitoring a change in the properties of the solution, such as a change in color or pH.
Titration instruments
Titration setup
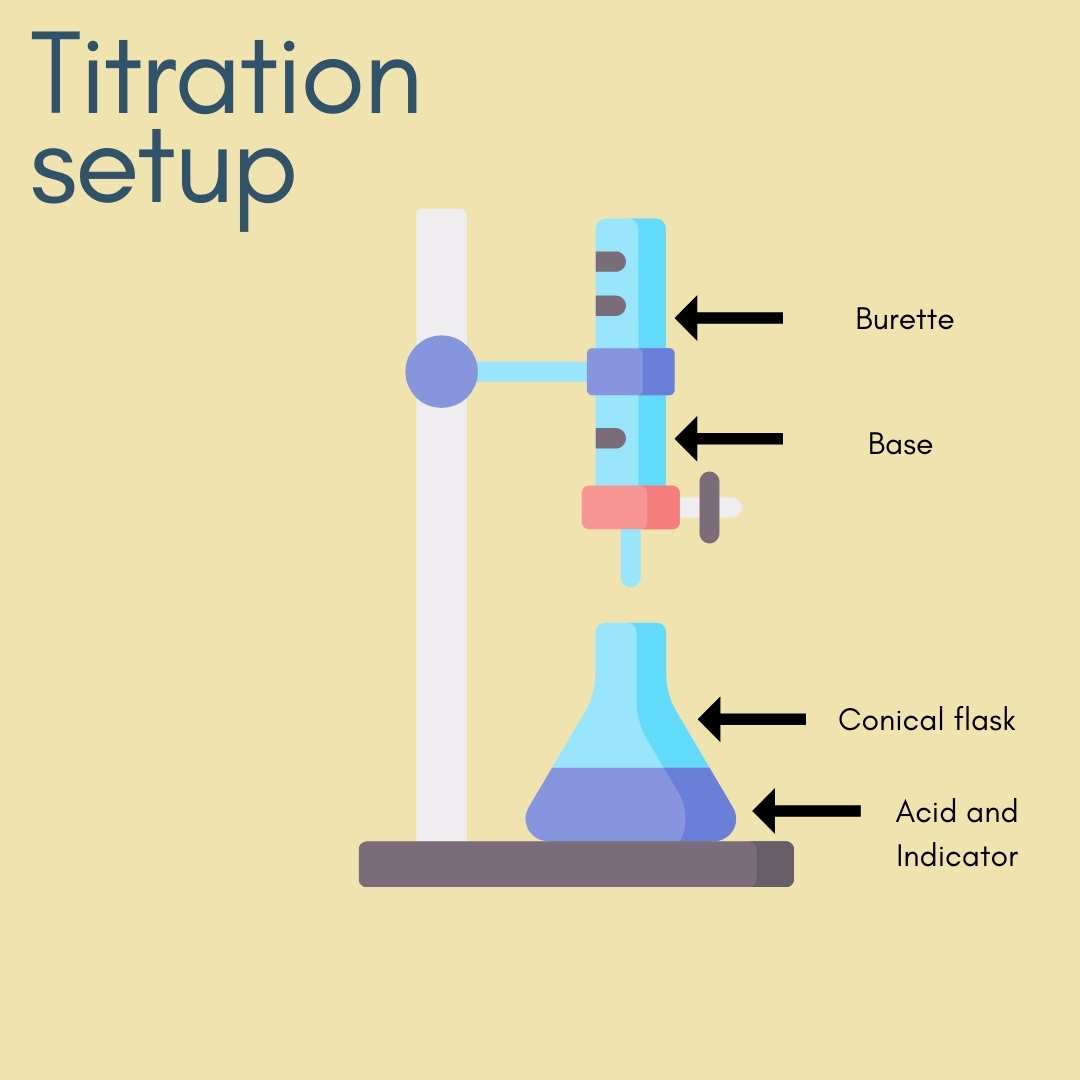
Titration setup picture

Acid base titration steps
Materials needed:
- Acid solution of known concentration
- Base solution of unknown concentration
- Indicator (e.g. phenolphthalein)
- Burette
- Pipette
- Conical flask
Steps:
- Rinse the burette with the base solution and fill it with the base solution up to the zero mark. Record the initial burette reading.
- Use a pipette to accurately measure a known volume of the acid solution into a conical flask.
- Add a few drops of indicator (e.g. phenolphthalein) to the conical flask.
- Place the conical flask under the burette.
- Slowly add the base solution from the burette to the acid solution in the conical flask, while swirling the flask. Stop adding base solution once the indicator changes color. Note the final burette reading.
- Record the volume of the base solution added to the acid solution by subtracting the initial burette reading from the final burette reading.
- Repeat the titration at least twice to obtain consistent results.
- Calculate the average volume of the base solution used.
- Use the balanced chemical equation for the reaction between the acid and base to calculate the moles of acid and base reacted.
- Use the number of moles of acid and base to calculate the concentration of the unknown base solution.
Titration steps

Titration equation
The equation used for the titration of a weak acid with a strong base is:
HA + OH– → A– + H2O
where HA is the weak acid and A– is the conjugate base of the acid. The endpoint of the titration is determined by using an indicator such as phenolphthalein, which changes color from colorless to pink when the pH of the solution reaches around 8.2.
Titration Curve
A titration curve is a graphical representation of the pH of a solution during a titration process. The x-axis of the titration curve represents the volume of the titrant added to the solution, while the y-axis represents the pH of the solution.
The titration curve of a weak acid-strong base titration has a characteristic shape, with a gradual increase in pH until the equivalence point is reached, followed by a sharp increase in pH. The steep increase in pH at the equivalence point indicates that the reaction is almost complete, and the solution has become neutral.
Equivalence Point Titration
The moles of the analyte being titrated are equivalent to the moles of the titrant given to the solution at the equivalence point of a titration.
For example, in a weak acid-strong base titration, the equivalence point is reached when all the acid has reacted with the base, and the solution has become neutral. At the equivalence point, the pH of the solution is equal to the pKa of the acid being titrated. The pH of the solution changes rapidly as the equivalence point is approached, indicating that the reaction is almost complete.
Equivalence Point on Titration Curve
The equivalence point on a titration curve is the point at which the pH of the solution changes rapidly. It is the point at which the analyte being titrated has reacted completely with the titrant, and the solution has become neutral. In a weak acid-strong base titration, the equivalence point is indicated by a sharp increase in pH. The equivalence point on the titration curve can be used to calculate the concentration of the analyte being titrated.
Titration Formula
The formula used to calculate the concentration of the analyte being titrated is:
C1V1 = C2V2
where C1 is the concentration of the analyte, V1 is the volume of the analyte, C2 is the concentration of the titrant, and V2 is the volume of the titrant added to the solution. This formula is used to calculate the concentration of the analyte at the equivalence point of the titration.
Titration Calculator
A titration calculator is a tool used to calculate the concentration of an analyte being titrated. The titration calculator requires the volume and concentration of the analyte, as well as the volume and concentration of the titrant. The calculator uses the titration formula to calculate the concentration of the analyte at the equivalence point.
Strong acid strong base titration
One of the most common types of titrations is the strong acid-strong base titration. In this type of titration, a strong acid is titrated with a strong base to determine the concentration of the acid. The reaction between the acid and base is typically a neutralization reaction, which produces water and a salt.
The equation for the reaction is:
acid + base → salt + water
During the titration, the base is gradually added to the acid until the equivalence point is reached, at which point all of the acid has reacted with the base. The equivalence point is typically detected using an indicator, such as phenolphthalein, which changes color at a specific pH. The amount of base required to reach the equivalence point can then be used to determine the concentration of the acid. The equation for the titration is written as:
Determination of the concentration of the acid
acid + base → salt + water
n acid V acid = n base V base
where n is the number of moles of acid or base and V is the volume of acid or base. The equation can be rearranged to solve for the unknown concentration of the acid or base.
titration is an essential technique in chemistry that allows scientists to determine the concentration of an unknown substance in a sample. The strong acid-strong base titration is one of the most common types of titrations and involves the gradual addition of a strong base to a strong acid to determine the concentration of the acid.



