Iron rusting is a chemical change that occurs when iron reacts with oxygen and water to form a compound called iron oxide or rust. This reaction is an example of an oxidation-reduction (redox) reaction, which involves the transfer of electrons between the reactants.
During the rusting process, iron atoms lose electrons to oxygen atoms, forming Fe2+ ions, while water molecules gain electrons to form hydroxide ions (OH–). These hydroxide ions then react with the Fe2+ ions to form rust.
Iron rusting is a chemical change
Iron rusting is a permanent and irreversible chemical change. Once the iron has rusted, it cannot be restored to its original state through physical means such as polishing or cleaning. This is because the rusting process alters the chemical structure of the iron, breaking down its original metallic properties and weakening its strength and durability. Therefore, it is important to take steps to prevent rusting from occurring, such as applying protective coatings or using rust-resistant materials.
The chemical equation for iron rusting
4Fe(s) + 3O2(g) + 6H2O(l) → 4Fe(OH)3(s)
This equation shows that four atoms of solid iron (Fe) react with three molecules of gaseous oxygen (O2) and six molecules of liquid water (H2O) to produce four molecules of solid iron(III) hydroxide (Fe(OH)3), which is the chemical formula for rust.
What causes iron rusting?
The main cause of iron rusting is exposure to moisture and oxygen. When iron is exposed to water and oxygen in the presence of an electrolyte, such as salt or acid, it undergoes a redox reaction that leads to rust formation. The electrolyte helps to speed up the reaction by facilitating the transfer of electrons between the iron and oxygen.
Other factors that can contribute to rust formation include high humidity, temperature, and pollutants in the environment. Therefore, it is important to store iron objects in dry and clean environments to prevent rusting.
Factors affecting iron rusting
Preventing iron rusting
Iron is a common metal used in various applications, but it is also prone to rusting when exposed to moisture and air. Rusting is a natural process where the iron reacts with oxygen to form iron oxide, which can lead to deterioration of the metal. Preventing iron rusting is essential to prolong the lifespan of iron objects. One way to protect iron from rusting is by applying a protective coating, such as paint or varnish, which creates a barrier between the metal and the environment. The coating acts as a shield that prevents moisture and air from reaching the iron surface, thus reducing the likelihood of rusting.
Another method to prevent rusting is by applying a rust inhibitor. Rust inhibitors work by slowing down the chemical reaction between iron and oxygen. They are typically applied as a thin film on the iron surface and can be used alone or in combination with a protective coating. Rust inhibitors can be found in various forms, such as sprays, paints, and oils.
Finally, keeping iron objects dry and clean is also an effective way to prevent rusting. Moisture and dirt can accelerate the rusting process, so it is important to keep iron surfaces dry and clean. If iron objects are exposed to moisture, they should be dried immediately to prevent rusting.
Science Experiments about Iron Rusting
Rusting is a common phenomenon that occurs when iron is exposed to moisture and oxygen for a prolonged period of time. It is a form of corrosion that can cause damage to iron and steel structures, as well as machinery and vehicles. Several science experiments can be performed to understand the process of rusting and its effects.
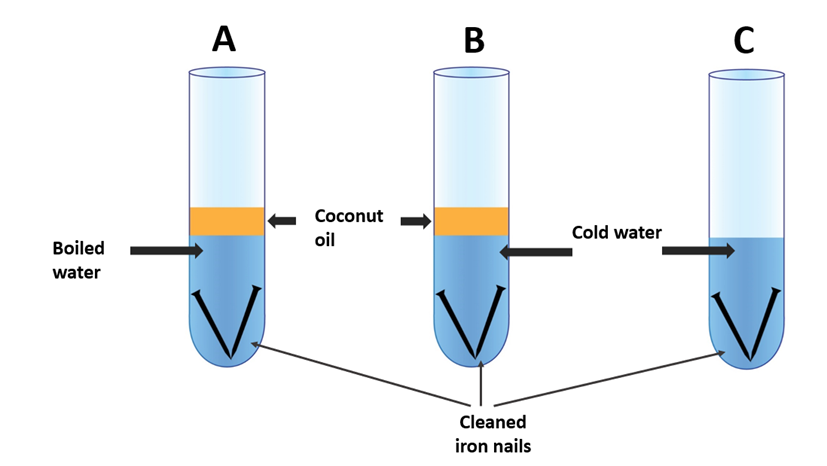
One experiment involves placing iron nails in a container filled with water and leaving them exposed to air for several days. Over time, the nails will start to rust, and the water will turn brown due to the formation of iron oxide. This experiment can be enhanced by adding different substances to the water, such as salt or vinegar, to observe the effect they have on the rate of rusting.
Another experiment involves measuring the rate of rusting in different environments, such as a dry room versus a humid room. This can be done by weighing iron nails before and after exposure to different environments and observing the difference in weight due to rusting.
Through these experiments, students can learn about the chemical reactions that occur during rusting and the importance of preventing rust in various applications, such as in the construction and maintenance of buildings and vehicles.
Iron Rusting experiment apparatus 1
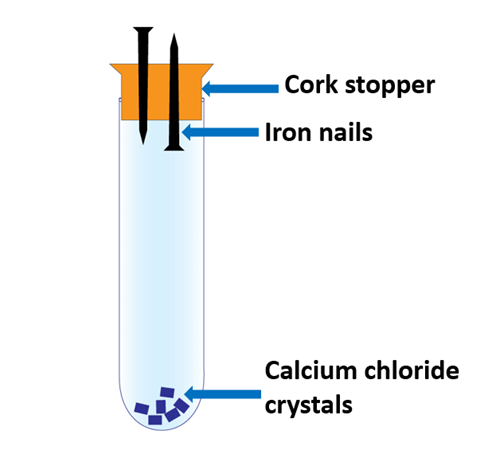
Iron Rusting experiment apparatus 2
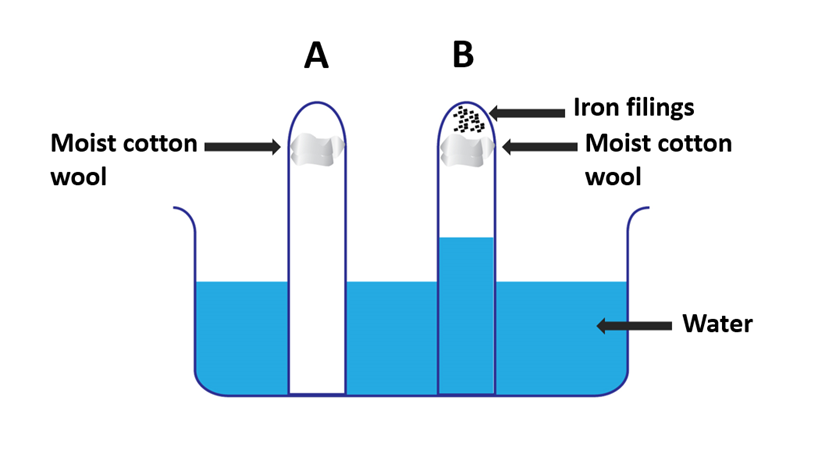
Iron Rusting experiment apparatus 3
Preventing iron rusting
Methods to protect iron from rusting
Iron can be protected from rusting using several methods.
Galvanization
Galvanization is a process of coating iron or steel with a layer of zinc to prevent rusting. Zinc is more reactive than iron and thus corrodes before the iron does. This process creates a barrier between the iron and the surrounding environment, preventing rust formation.
Painting
Painting is another effective way to protect iron from rusting. A coat of paint creates a physical barrier between the iron and the surrounding air, preventing moisture and oxygen from coming into contact with the metal. This method is particularly useful for iron objects that are exposed to the elements.
Powder coating
Powder coating is a process that involves applying a layer of electrostatically charged powder to a metal surface. The coated metal is then baked in an oven, which causes the powder to melt and form a hard, durable layer. Powder coating provides excellent protection against rust and other forms of corrosion.
Oil or wax coating
Applying a thin layer of oil or wax to iron surfaces can help prevent rust formation. The oil or wax creates a barrier that prevents moisture and oxygen from coming into contact with the metal. This method is particularly useful for small iron objects, such as hand tools.
Cathodic protection
Cathodic protection is a method of protecting iron from rusting by using a sacrificial anode. A sacrificial anode, typically made of zinc or magnesium, is connected to the iron object. The anode corrodes instead of the iron, protecting the metal from rusting.
Keeping it dry
Keeping iron objects dry is the simplest way to prevent rusting. Moisture is a key factor in rust formation, so storing iron objects in a dry place or using a dehumidifier can help prevent rust formation.
Metal tarnish
Does Metal Tarnish?
Yes, many metals can tarnish when exposed to air or other environmental factors. Tarnishing is a form of corrosion that causes a discoloration or dullness on the surface of metals, such as silver, brass, and copper. The process of tarnishing is usually caused by a reaction between the metal and chemicals present in the environment, such as oxygen, sulfur, and moisture.
The speed at which a metal tarnishes depends on the metal type and the environment it is exposed to. Some metals tarnish more quickly than others, such as copper, which can develop a greenish patina in just a few hours of exposure to air. Other metals, such as stainless steel, are resistant to tarnishing due to the protective layer of chromium oxide that forms on the surface.
Metal tarnish remover
There are several methods and products that can be used to remove tarnish from metal, depending on the type of metal and the severity of the tarnish.
Lemon juice and baking soda
Mix lemon juice and baking soda to make a paste. Apply the paste to the tarnished metal and let it sit for a few minutes before wiping it off with a soft cloth. Rinse the metal with water and dry it thoroughly.
Vinegar and salt
Mix vinegar and salt to make a paste. Apply the paste to the tarnished metal and let it sit for a few minutes before wiping it off with a soft cloth. Rinse the metal with water and dry it thoroughly.
Commercial tarnish remover
There are many commercial tarnish removers available that are specifically designed for certain types of metal.
Aluminum foil and baking soda
Line a pan with aluminum foil and fill it with hot water. Add baking soda and stir until it dissolves. Place the tarnished metal in the solution and let it sit for a few minutes. Rinse the metal with water and dry it thoroughly.