What is Activity series?
The activity series is a list of elements in order of their relative reactivity. It is used to predict the products of single displacement reactions and to help identify the oxidizing agent and reducing agent in a redox reaction.
The activity series is based on the observation that some elements are more likely to lose electrons and be oxidized (become positive ions) while others are more likely to gain electrons and be reduced (become negative ions). The most reactive elements are at the top of the activity series, while the least reactive elements are at the bottom.
For example, the activity series for some common elements is:
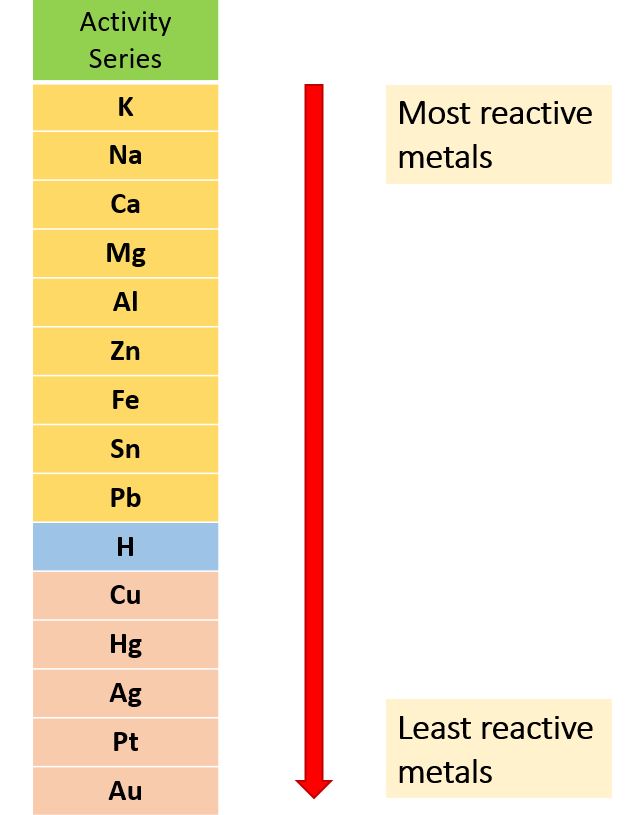
Potassium > Sodium > Calcium > Magnesium > Aluminum > Zinc > Iron > Nickel > Tin > Lead > Hydrogen > Copper > Silver > Gold > Platinum
This means that potassium is the most reactive element on the list and is more likely to lose electrons and be oxidized. On the other hand, platinum is the least reactive element on the list and is less likely to lose electrons and be oxidized.
The activity series can be used to predict the products of single displacement reactions, where one element is replaced by another element in a compound. For example, if zinc metal is placed in a solution of copper(II) sulfate, the zinc will displace the copper and form zinc sulfate, because zinc is higher in the activity series than copper. The reaction can be represented by the following equation:
Zn(s) + CuSO4(aq) –> ZnSO4(aq) + Cu(s)
The activity series can also be used to identify the oxidizing agent and reducing agent in a redox reaction. The oxidizing agent is the element that is being reduced (gaining electrons) and the reducing agent is the element that is being oxidized (losing electrons). In the example above, the copper(II) sulfate is the oxidizing agent and the zinc is the reducing agent
The activity series of metals is a list of metals arranged in order of decreasing ease of oxidation, or the ability to lose electrons. The activity of a metal can be determined by its tendency to form a positive ion (cation) by losing electrons to a nonmetal.
The activity series is used to predict the products of displacement reactions and to identify the more reactive element in a redox reaction. The activity series can also be used to predict the products of reactions between metals and their compounds.
Here is a list of common metals in the activity series, from most active (most likely to lose electrons) to least active (least likely to lose electrons):
-
-
- Potassium
- Sodium
- Calcium
- Magnesium
- Aluminum
- Zinc
- Iron
- Nickel
- Tin
- Lead
-
It’s important to note that the activity series is not a fixed list and can vary depending on the specific conditions of a reaction. The position of a metal on the activity series is determined by its standard reduction potential, which is a measure of the tendency of a metal to gain or lose electrons. The activity series is a useful tool for predicting the products of redox reactions, but it should be used with caution, as other factors such as the nature of the reactants and the presence of catalysts can affect the outcome of a reaction.
What does an activity series do?
The activity of an element is a measure of its tendency to participate in chemical reactions, and the activity series is used to predict how elements will behave in a chemical reaction. The activity series is based on the idea that some elements are more reactive than others, and that the reactivity of an element can be predicted by its position in the series. The activity series is often used in chemistry to predict the products of a chemical reaction and to determine which elements will be oxidized or reduced in a reaction.
What is known as activity series?
An activity series is a list of elements arranged in order of their relative reactivity.
What determines the activity series?
The activity series is a list of elements arranged in order of their relative reactivity. It is used to predict the products of single replacement reactions, in which one element is replaced by another element in a compound. The activity of an element is determined by its ability to lose electrons and form positive ions, or cations. More active elements are able to more readily lose electrons and form cations, while less active elements are less likely to do so.
Elements that are more likely to lose electrons and form cations are higher up in the activity series, while elements that are less likely to lose electrons and form cations are lower down in the activity series. The activity series is often represented as a list, with the most active elements at the top and the least active elements at the bottom.
The activity series is determined by the relative stability of the ions that the elements can form. Elements that form more stable ions are more active, while elements that form less stable ions are less active. Factors that contribute to the stability of an ion include the size of the ion, the charge on the ion, and the arrangement of the electrons in the ion.
In general, elements that are smaller and have a higher charge are more stable, while elements that are larger and have a lower charge are less stable. Similarly, ions that have a filled valence shell (that is, a stable electron configuration) are more stable than ions that do not. These factors all contribute to the activity of an element and determine its position in the activity series.
How do you memorize activity series?
There are a few different ways you can try to memorize the activity series:
Use mnemonic devices: Mnemonic devices are memory aids that use a rhyme, acronym, or other memorable phrase to help you remember a list of items. For example, you could try using the acronym to remember the activity series (Lithium, Aluminum, Sodium, Potassium, Calcium, Iron, Nickel, Copper, Zinc, Gold, Silver, Mercury).
Practice recalling the activity series: Try writing out the activity series from memory and then checking your work against a reference. You can do this multiple times until you feel comfortable recalling the activity series on your own.
Use visual aids: You might find it helpful to create a visual representation of the activity series, such as a mind map or a concept map. This can help you to understand the relationships between the elements and make it easier to remember their order.
Review regularly: Finally, it’s important to review the activity series regularly to help it sink in. Try reviewing it every day or every other day until you feel confident that you can recall it accurately.
It’s also worth noting that memorizing the activity series is not always necessary, as you can always look it up if you need to. However, being able to recall the activity series can be helpful in certain situations, such as when you are working on a chemistry problem and don’t have access to a reference.
When should you use the activity series?
The activity series is a useful tool for predicting the products of single replacement reactions, in which one element is replaced by another element in a compound. In a single replacement reaction, an element from a compound is replaced by an element from a pure substance. The activity series can be used to determine whether a single replacement reaction is likely to occur, and if so, which element will be replaced.
To use the activity series, you first need to determine the reactivity of the two elements involved in the reaction. The more active element will replace the less active element in the compound. For example, if you have a compound containing zinc and you want to know if it will react with a solution of copper(II) sulfate, you can consult the activity series to see which element is more reactive. If zinc is higher in the activity series than copper, then the zinc will replace the copper in the compound.
The activity series can also be used to predict the products of redox reactions, which involve a transfer of electrons from one species to another. In a redox reaction, the species that gains electrons is said to be reduced, while the species that loses electrons is said to be oxidized. The activity series can be used to determine which element is more likely to be oxidized and which element is more likely to be reduced.
It’s worth noting that the activity series is not a precise prediction of the products of a chemical reaction, as there are many other factors that can affect the outcome of a reaction. However, it can be a useful tool for predicting the general trend of a reaction and can help you to understand the reactivity of different elements.
An element in the activity series can replace any element
The activity series is a list of elements arranged in order of their relative reactivity. It is used to predict the products of single replacement reactions, in which one element is replaced by another element in a compound. In a single replacement reaction, an element from a compound is replaced by an element from a pure substance. The activity series can be used to determine which element is more likely to replace the other in the compound.
According to the activity series, an element that is higher in the series is more reactive and is more likely to replace an element that is lower in the series. For example, if you have a compound containing zinc and you want to know if it will react with a solution of copper(II) sulfate, you can consult the activity series to see which element is more reactive. If zinc is higher in the activity series than copper, then the zinc will replace the copper in the compound.
It’s worth noting that the activity series is not a precise prediction of the products of a chemical reaction, as there are many other factors that can affect the outcome of a reaction. However, it can be a useful tool for predicting the general trend of a reaction and can help you to understand the reactivity of different elements.
Does the activity series apply to double replacement reactions?
The activity series is a list of elements arranged in order of their relative reactivity. It is primarily used to predict the products of single replacement reactions, in which one element is replaced by another element in a compound. In a single replacement reaction, an element from a compound is replaced by an element from a pure substance.
The activity series is less useful for predicting the products of double replacement reactions, in which the ions in two compounds are exchanged to form two new compounds. In a double replacement reaction, the reactivity of the elements is not as important as the charges on the ions involved in the reaction.
To predict the products of a double replacement reaction, you need to consider the charges on the ions involved in the reaction. If the ions have opposite charges, then a double replacement reaction is likely to occur. The resulting products will be two new compounds, with the ions from the reactants swapping places.
For example, consider the double replacement reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH). The ions in these compounds are H+ and Cl-, on the one hand, and Na+ and OH-, on the other. Since the ions have opposite charges, a double replacement reaction is likely to occur. The resulting products will be sodium chloride (NaCl) and water (H2O).
It’s worth noting that the activity series can still be useful in predicting the products of double replacement reactions in some cases. For example, if one of the reactants is a compound containing an element that is very low in the activity series (such as copper), then it is unlikely to react with any other element. In this case, the activity series can be used to predict that no reaction will occur. However, in most cases, the charges on the ions are the more important factor in predicting the products of a double replacement reaction.
What is the correct order of activity series?
The activity series is a list of elements arranged in order of their relative reactivity. It is used to predict the products of single replacement reactions, in which one element is replaced by another element in a compound. The activity of an element is determined by its ability to lose electrons and form positive ions, or cations. More active elements are able to more readily lose electrons and form cations, while less active elements are less likely to do so.
Here is the correct order of the activity series:
It’s worth noting that the activity series is not a precise prediction of the products of a chemical reaction, as there are many other factors that can affect the outcome of a reaction. However, it can be a useful tool for predicting the general trend of a reaction and can help you to understand the reactivity of different elements.
Is activity series only for single replacement?
The activity series is primarily used to predict the products of single replacement reactions, in which one element is replaced by another element in a compound. In a single replacement reaction, an element from a compound is replaced by an element from a pure substance. The activity series can be used to determine which element is more likely to replace the other in the compound.
According to the activity series, an element that is higher in the series is more reactive and is more likely to replace an element that is lower in the series. For example, if you have a compound containing zinc and you want to know if it will react with a solution of copper(II) sulfate, you can consult the activity series to see which element is more reactive. If zinc is higher in the activity series than copper, then the zinc will replace the copper in the compound.
It’s worth noting that the activity series is not a precise prediction of the products of a chemical reaction, as there are many other factors that can affect the outcome of a reaction. However, it can be a useful tool for predicting the general trend of a reaction and can help you to understand the reactivity of different elements.
The activity series is less useful for predicting the products of double replacement reactions, in which the ions in two compounds are exchanged to form two new compounds. In a double replacement reaction, the reactivity of the elements is not as important as the charges on the ions involved in the reaction. To predict the products of a double replacement reaction, you need to consider the charges on the ions involved in the reaction. If the ions have opposite charges, then a double replacement reaction is likely to occur.
Five facts about activity series
The activity series is a list of elements arranged in order of their relative reactivity. It is used to predict the products of single replacement reactions, in which one element is replaced by another element in a compound.
The activity of an element is determined by its ability to lose electrons and form positive ions, or cations. More active elements are able to more readily lose electrons and form cations, while less active elements are less likely to do so.
Elements that are more likely to lose electrons and form cations are higher up in the activity series, while elements that are less likely to lose electrons and form cations are lower down in the activity series.
The activity series is determined by the relative stability of the ions that the elements can form. Elements that form more stable ions are more active, while elements that form less stable ions are less active.
The activity series is not a precise prediction of the products of a chemical reaction, as there are many other factors that can affect the outcome of a reaction. However, it can be a useful tool for predicting the general trend of a reaction and can help you to understand the reactivity of different elements.
Uses of activity series
The activity series might help you discover the measures to take when storing metals. High-reactivity metals such as sodium (Na), potassium (K), and calcium (Ca) should be stored in liquids such as kerosene and liquid paraffin. Because of their strong reactivity with air, they react with the components in air if left exposed.
The activity series might help you find solutions to prevent metal corrosion. Keeping iron in contact with metals that are more reactive than iron, such as zinc and magnesium, is one example.
The activity series aids in the selection of metals for the construction of electrochemical cells.
The activity series can be used to determine the best ways for extracting metals. Metal extraction is the process of separating an element from a natural resource that contains that metal. The occurrence of metals varies depending to their reactivity.
Reactive metals such as sodium (Na) and potassium (K) cannot be considered native metals in nature. They are found in the environment as very stable ionic compounds. Electrolysis, a reliable metal extraction procedure, must be used to extract them. Electrolysis of their fused (molten) chloride is employed to remove these metals.
Metals with a moderate degree of reactivity, such as iron (Fe), tin (Sn), zinc (Zn), and lead (Pb), are extracted by reducing their compounds with other elements or compounds.
Low-reactivity metals such as silver (Ag), gold (Au), and platinum (Pt) occur naturally as native metals combined with other compounds. The physical processes used to separate the mixtures extract them.
As a result, metals near the top of the activity range are extracted using strong extraction methods such as electrolysis. At the bottom of the activity series, simpler physical processes are used to extract metals.