Introduction
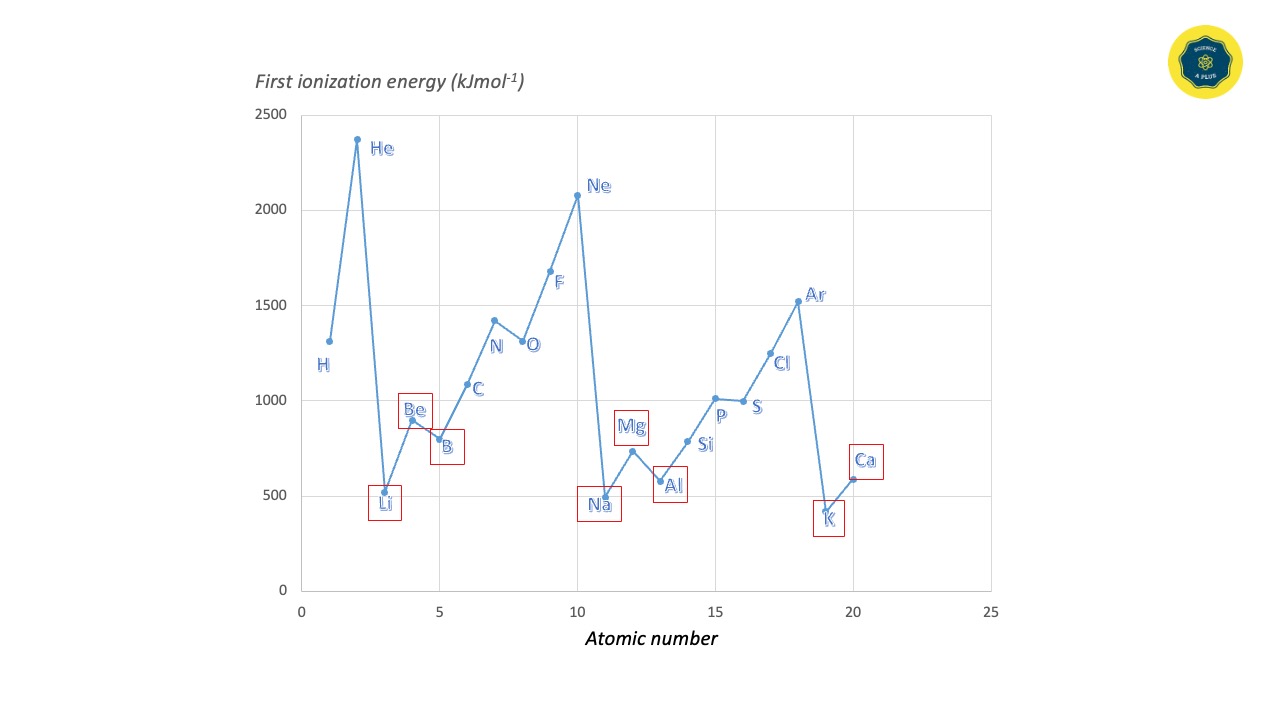
Matters are composed of pure and non-pure substances. Pure substances are found as elements and compounds. All elements that are found on the Earth are mentioned in the periodic table. Out of these elements, metals are substances with specific features. All the metals on the periodic table are shown in the following chart with their symbolic name and atomic number.
The metals in the periodic table can be categorized into several groups based on their chemical properties. They are alkali metals, alkaline earth metals, transition metals, post-transition metals, lanthanides, and actinides. Here are some examples of metals belonging to each category.
Alkali metals – Sodium, Potassium, Lithium
Alkaline Earth Metals – Calcium, Magnesium, Beryllium
Transition Metals – Iron, Cobalt, Copper
Post Transition Metals – Aluminium, Lead, Zinc
Lanthanides – Lanthanum, Terbium, Europium
Actinides – Uranium, Plutonium, Thorium
In other classifications metals are classified into different categories with the basis of some specific criteria such as their magnetic behavior, brittleness, refraction ability, color, density, conductivity, reactivity, etc.
All these metals can be found in the periodic table from the left side to the middle and they are marked with a specific coloration. Furthermore, there are two separate rows below the main table that contains metals.
The following table shows the names of the metals and their symbols. It is in the order of the atomic number. The periodic table is also arranged in the order of the atomic number of the elements.
| Name of the metal and the atomic number | Symbol of the metal |
| 3 – Lithium | Li |
| 4 – Beryllium | Be |
| 11 – Sodium | Na |
| 12 – Magnesium | Mg |
| 13 – Aluminium | Al |
| 19 – Potassium | K |
| 20 – Calcium | Ca |
| 21 – Scandium | Sc |
| 22 – Titanium | Ti |
| 23 – Vanadium | V |
| 24 – Chromium | Cr |
| 25 – Manganese | Mn |
| 26 – Iron | Fe |
| 27 – Cobalt | Co |
| 28 – Nickel | Ni |
| 29 – Copper | Cu |
| 30 – Zinc | Zn |
| 31 – Gallium | Ga |
| 37 – Rubidium | Rb |
| 38 – Strontium | Sr |
| 39 – Yttrium | Y |
| 40 – Zirconium | Zr |
| 41 – Niobium | Nb |
| 42 – Molybdenum | Mo |
| 43 – Technetium | Tc |
| 44 – Ruthenium | Ru |
| 45 – Rhodium | Rh |
| 46 – Palladium | Pd |
| 47 – Silver | Ag |
| 48 – Cadmium | Cd |
| 49 – Indium | In |
| 50 – Tin | Sn |
| 55 – Cesium | Cs |
| 56 – Barium | Ba |
| 57 – Lanthanum | La |
| 58 – Cerium | Ce |
| 59 – Praseodymium | Pr |
| 60 – Neodymium | Nd |
| 61 – Promethium | Pm |
| 62 – Samarium | Sm |
| 63 – Europium | Eu |
| 64 – Gadolinium | Gd |
| 65 – Terbium | Tb |
| 66 – Dysprosium | Dy |
| 67 – Holmium | Ho |
| 68 – Erbium | Er |
| 69 – Thulium | Tm |
| 70 – Ytterbium | Yb |
| 71 – Lutetium | Lu |
| 72 – Hafnium | Hf |
| 73 – Tantalum | Ta |
| 74 – Tungsten | W |
| 75 – Rhenium | Re |
| 76 – Osmium | Os |
| 77 – Iridium | Ir |
| 78 – Platinum | Pt |
| 79 – Gold | Au |
| 80 – Mercury | Hg |
| 81 – Thallium | Tl |
| 82 – Lead | Pb |
| 83 – Bismuth | Bi |
| 84 – Polonium | Po |
| 87 – Francium | Fr |
| 88 – Radium | Ra |
| 89 – Actinium | Ac |
| 90 – Thorium | Th |
| 91 – Protactinium | Pa |
| 92 – Uranium | U |
| 93 – Neptunium | Np |
| 94 – Plutonium | Pu |
| 95 – Americium | Am |
| 96 – Curium | Cm |
| 97 – Berkelium | Bk |
| 98 – Californium | Cf |
| 99 – Einsteinium | Es |
| 100 – Fermium | Fm |
| 101 – Mendelevium | Md |
| 102 – Nobelium | No |
| 103 – Lawrencium | Lr |
| 104 – Rutherfordium | Rf |
| 105 – Dubnium | Db |
| 106 – Seaborgium | Sg |
| 107 – Bohrium | Bh |
| 108 – Hassium | Hs |
| 109 – Meitnerium | Mt |
| 110 – Darmstadtium | Ds |
| 111 – Roentgenium | Rg |
| 112 – Copernicium | Cn |
| 113 – Nihonium | Nh |
| 114 – Flerovium | Fl |
| 115 – Moscovium | Mc |
| 116 – Livermorium | Lv |
The above metals have their unique features as well as common features. These features help to differentiate metals from non-metals.
Here is a list of common physical properties of the metals.
Common physical properties of metals
- Lustrous nature – shiny
- Produce sonorous sound – ringing sound
- Good heat conductors
- Good electric conductors
- Solids at room temperature (except mercury)
- Can be drawn into thin wires without breaking – ductility
- Can be hammered into thin sheets without breaking – malleability
Common chemical properties of metals
- Ability to form cations by losing electrons
- Show relatively low electronegativity
- Having low ionization energy
- Metal oxides are basic oxides
- Metal oxides form basic solutions with water
Uses of metals
- Taken as raw materials for constructions
- Provide nutrients for the human body and plants
- Used as raw material in several industries
- Used in extracting other metals