Beryllium is a rare and highly toxic metal with remarkable physical and chemical properties.
Beryllium symbol: Be
Physical properties of Beryllium element:
Beryllium is a hard, silvery-gray metal that is brittle and has a low density.
It has a high melting point and is a good conductor of electricity.
Beryllium is very corrosion resistant and is unaffected by concentrated nitric or hydrochloric acid.
Chemical properties of Beryllium element:
Beryllium is highly reactive and readily forms compounds with a wide range of other elements and compounds.
It strongly reacts with acids, producing beryllium salts.
Beryllium can form compounds with both nonmetals and metals, and can exist in various oxidation states.
Beryllium valence electrons:
How many valence electrons does beryllium have?
Beryllium has a valency of +2, which means it loses two electrons in chemical reactions to form a stable cation.
Beryllium electron configuration:
The electronic configuration of beryllium is 1s2 2s2, which means it has two electrons in the first energy level and two electrons in the second energy level.
Beryllium uses:
Beryllium is used in a number of high-tech applications, including the production of nuclear reactors, X-ray machines, and spacecraft.
It is also used in the production of lightweight alloys for the aerospace industry.
Because of its lightweight and high strength, beryllium is sometimes utilized in golf clubs and other sporting equipment.
Beryllium is not used in medicine due to its highly toxic nature. In fact, beryllium exposure can cause a serious lung disease called berylliosis.
Reactions of Beryllium element with other elements:
Beryllium reacts with a wide range of elements and compounds, including acids, halogens, and oxygen.
Equation for reaction of Beryllium with Hydrochloric acid
Beryllium + Hydrochloric acid → Beryllium chloride + Hydrogen gas
Balanced equation for reaction of Beryllium with Hydrochloric acid
Be + 2HCl → BeCl2 + H2
Equation for reaction of Beryllium with Nitric acid
Beryllium + Nitric acid → Beryllium nitrate + Nitrogen dioxide gas + Water
Balanced equation for reaction of Beryllium with Nitric acid
2Be + 4HNO3 → 2Be(NO3)2 + 2NO2 + 2H2O
Equation for reaction of Beryllium with Oxygen
Beryllium + Oxygen → Beryllium oxide
Balanced equation for reaction of Beryllium with Oxygen
2Be + O2 → 2BeO
Equation for reaction of Beryllium with Fluorine gas
Beryllium + Fluorine gas → Beryllium fluoride
Balanced equation for reaction of Beryllium with Fluorine gas
Be + F2 → BeF2
Equation for reaction of Beryllium with Chlorine gas
Beryllium + Chlorine gas → Beryllium chloride
Balanced equation for reaction of Beryllium with Chlorine gas
Be + Cl2 → BeCl2
Equation for reaction of Beryllium with Hydrochloric acid
Beryllium + Hydrochloric acid → Beryllium chloride + Hydrogen gas
Balanced equation for reaction of Beryllium with Hydrochloric acid
Be + 2HCl → BeCl2 + H2
Beryllium Oxide Formula:
The chemical formula for beryllium oxide is BeO.
Beryllium Chloride Formula:
The chemical formula for beryllium chloride is BeCl2.
Beryllium Hydroxide Formula:
The chemical formula for beryllium hydroxide is Be(OH)2. It is a white, crystalline solid that is insoluble in water and has a high melting point.
Beryllium Hydroxide uses
Beryllium hydroxide is used as a precursor to beryllium oxide, which is commonly used in the production of ceramics and electronic components.
Beryllium Oxide:
Beryllium oxide is an inorganic compound with the chemical formula BeO. It is a white, crystalline solid that is insoluble in water and has a high melting point.
Beryllium Oxide uses
Beryllium oxide is commonly used as a ceramic material due to its high thermal conductivity, electrical insulation properties, and resistance to thermal shock.
Beryllium Copper:
Beryllium copper is an alloy made of beryllium and copper. It is a high-strength, non-magnetic material that is resistant to corrosion and wear.
Beryllium Copper uses
Beryllium copper is commonly used in aerospace and defense applications, as well as in electrical and electronic components.
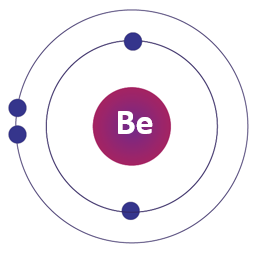
Beryllium Bohr Model:
The Bohr model is a simplified representation of an atom in which electrons orbit the nucleus in specific energy levels. The Bohr model of beryllium shows the nucleus with four protons and usually four neutrons, with two electrons in the first energy level and two electrons in the second energy level.
Beryllium Atom:
A beryllium atom has four protons in its nucleus and usually four neutrons, with two electrons in the first energy level and two electrons in the second energy level. Beryllium is a rare, lightweight metal with a high melting point and good electrical conductivity.
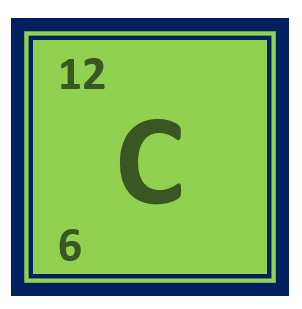
Beryllium Periodic Table:
Beryllium is a chemical element with the symbol Be and atomic number 4. It is located in Group 2, Period 2 of the periodic table. Beryllium is a rare, lightweight metal with a high melting point and good electrical conductivity.
Beryllium Protons Neutrons Electrons:
Beryllium (Be) has 4 protons, 4 electrons, and its most common isotope (Be-9) has 5 neutrons. The number of neutrons can vary depending on the isotope of beryllium.
Protons: 4
Electrons: 4
Neutrons: 5 (for the most common isotope, Be-9)
How Many Neutrons Does Beryllium Have:
The most common isotope of Beryllium is Be-9, which has 4 protons and 5 neutrons. However, Beryllium has a few other isotopes, such as Be-7, Be-8, Be-10, and Be-11, which have different numbers of neutrons.