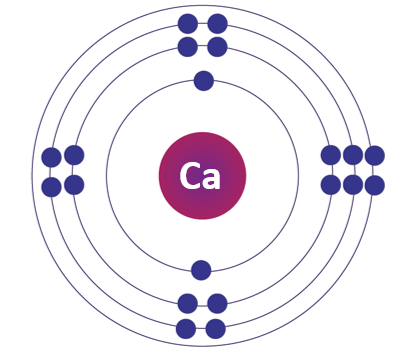
Calcium element
Chemical Properties of Calcium:
- Calcium is a reactive metal that easily forms compounds with other elements; for example, it reacts with oxygen to form calcium oxide, and with water to form calcium hydroxide and hydrogen gas.
- It also combines with acids to produce calcium salts and hydrogen gas.
- Calcium is an excellent reducing agent, which means it can donate electrons to other elements in order to reduce their positive charge.
Physical Properties of Calcium:
- Calcium is a silvery-white metal with a pliable texture.
- It is a good electrical conductor with a high melting and boiling point.
- It has a hexagonal close-packed crystal structure and is relatively dense.
Chemical Properties of Calcium in a table:
| Property | Description |
| Atomic symbol | Ca |
| Atomic number | 20 |
| Atomic weight | 40.078 g/mol |
| Electron configuration | [Ar]4s² |
| Oxidation states | +2 |
| Density | 1.54 g/cm³ (solid), 1.55 g/cm³ (liquid) |
| Melting point | 842°C |
| Boiling point | 1,484°C |
| Heat of fusion | 8.54 kJ/mol |
| Heat of vaporization | 155 kJ/mol |
| Specific heat | 0.647 J/g·K at 25 °C |
| Electronegativity | 1.00 (Pauling scale) |
| Ionization energy | First: 589.8 kJ/mol, Second: 1,145 kJ/mol, Third: 4,916 kJ/mol |
| Reactivity | Calcium readily reacts with water to produce calcium hydroxide and hydrogen gas. It also reacts with oxygen to form calcium oxide (quicklime) and nitrogen to form calcium cyanamide. Calcium is used to produce alloys, as a reducing agent in metallurgy, and to remove impurities from molten metals. It is also used in the production of cement and as a dietary supplement. |
Physical Properties of Calcium in a table:
| Property | Description |
| Atomic symbol | Ca |
| Atomic number | 20 |
| Atomic weight | 40.078 g/mol |
| Electron configuration | [Ar]4s² |
| Physical state | Solid |
| Color | Silver-grey |
| Odor | Odorless |
| Density | 1.55 g/cm³ (at room temperature) |
| Melting point | 842°C |
| Boiling point | 1,484°C |
| Crystal structure | Face-centered cubic (fcc) |
| Solubility | Soluble in water, reacts with water to produce calcium hydroxide and hydrogen gas. |
| Magnetic properties | Diamagnetic |
| Thermal conductivity | 201 W/(m·K) at 300 K |
| Electrical conductivity | 29.6×10^6 S/m at room temperature |
| Hardness | 1.75 on Mohs scale |
| Malleability | Malleable |
| Ductility | Ductile |
| Tensile strength | 41 MPa |
| Young’s modulus | 20 GPa |
| Shear modulus | 7.4 GPa |
| Bulk modulus | 17 GPa |
| Poisson’s ratio | 0.31 |
| Specific heat capacity | 0.647 J/(g·K) at 25 °C |
| Thermal expansion | 22.3 × 10^−6 K^−1 (at 25 °C) |
Valency of Calcium:
Calcium has a valency of +2, meaning it typically loses two electrons to form a stable ion.
Electronic Configuration of Calcium:
Its electronic configuration is [Ar] 4s2, indicating it has two valence electrons in the outermost shell.
Reactions with other Elements:
Calcium readily reacts with halogens such as chlorine and fluorine to form ionic compounds.
It also forms compounds with oxygen, sulfur, nitrogen, and many other non-metal elements.
Industrial Uses of Calcium:
- Calcium is a mineral that is widely used in the production of steel, aluminum, and other metals.
- It is used in the extraction of metals from ores as a reducing agent.
- A common calcium compound, calcium carbonate, is used as a filler in plastics, paints, and other products.
- Calcium oxide, also known as quicklime, is used in the production of cement and other building materials.
Calcium pool
Medical Uses of Calcium:
- Calcium is a necessary nutrient for human and animal health, as well as strong bones and teeth.
- Calcium supplements are used to treat or prevent calcium deficiency and other health problems.
- Calcium is also used in medical imaging procedures like X-rays and CT scans.
Calcium Hydroxide:
Chemical formula for Calcium Hydroxide: Ca(OH)2
Features of Calcium Hydroxide:
- It is known as hydrated lime or slaked lime.
- It is a white powder that is odorless and slightly alkaline.
Uses of Calcium Hydroxide:
It is used in many different applications, including water treatment, construction, and food production.
Calcium Phosphate:
Chemical formula for Calcium Phosphate: Ca3(PO4)2
Facts about Calcium Phosphate:
- A group of compounds that contain calcium cations and phosphate anions.
- It is the primary component of bone and teeth, and is essential for their growth and maintenance.
Uses of Calcium Phosphate:
Calcium phosphate is also used as a food additive and a phosphorus source in fertilizers.
Calcium Nitrate:
Chemical formula for Calcium Nitrate: Ca(NO3)2
Features of Calcium Nitrate:
- It is a white, crystalline compound that is extremely water soluble.
Uses of Calcium Nitrate:
It is used in agriculture as a fertilizer and as a component in some explosives and pyrotechnics.
Calcium Acetate:
Chemical formula for Calcium Acetate: Ca(C2H3O2)2
Features of Calcium Acetate:
- It is a white, crystalline compound that is water soluble.
Uses of Calcium Acetate:
It is used in medicine to treat high phosphate levels in the blood and as a food additive.
Calcium Sulfate:
Chemical formula for Calcium Sulfate: CaSO4
Features of Calcium Sulfate:
- It is a white, odorless powder that is slightly water soluble.
Uses of Calcium Sulfate:
It is used in the construction industry as a component of plaster and drywall, as well as a food additive.
Calcium Oxalate Crystals in Urine:
Calcium oxalate crystals form in the urine and are small, sharp crystals.
They are a common cause of kidney stones, which can be painful and cause other problems.
Dehydration, high calcium or oxalate levels in the diet, and certain medical conditions can all increase the risk of developing calcium oxalate crystals.
Formula for Calcium Fluoride: CaF2
Formula for Calcium Nitride: Ca3N2
Formula for Calcium Nitrate: Ca(NO3)2
Chemical formula for Calcium Chloride: CaCl2
Chemical formula for Calcium Phosphate: Ca3(PO4)2
Formula for Calcium Hydroxide: Ca(OH)2
Formula for Calcium Oxide: CaO