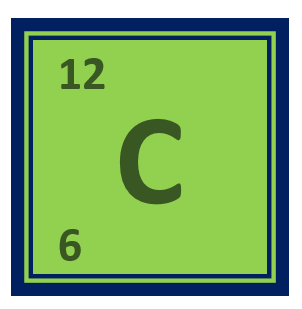
Carbon is a chemical element with the symbol C and the atomic number is 6. It is a nonmetallic element and the basis of all known life on Earth.
Physical properties of carbon:
Carbon has multiple allotropes, including graphite, diamond, and fullerenes.
Graphite is a soft, black, and slippery material that is used in pencils, lubricants, and electrodes.
The hardest known material is diamond, which is used in jewelry, cutting tools, and as an abrasive.
Carbon has a relatively low melting point of 3500°C and boiling point of 4827°C.
Chemical properties of carbon:
Carbon has the ability to make covalent connections with other elements as well as with itself, allowing it to build lengthy chains and complex structures.
Carbon has a high electronegativity, which implies it actively attracts electrons.
carbon element valence electrons
It has four valence electrons and has the ability to make up to four covalent bonds.
Electronic configuration of carbon:
Carbon has an electronic configuration of 1s²2s²2p², with four valence electrons in the 2s and 2p orbitals.
Uses of carbon:
Carbon is used in the production of steel and other alloys.
It is utilized in the manufacturing of activated carbon, which is used in air and water purification systems as a filter and adsorbent.
Carbon is used to make carbon black, which is a pigment and reinforcing ingredient in rubber products.
It is also used in the production of carbon fiber, which is used in high-strength composites for aerospace, sports equipment, and other applications.
Reactions of carbon with other elements:
Carbon reacts with oxygen to form carbon dioxide (CO2) and with water to form carbonic acid (H2CO3).
It can also react with halogens to form carbon halides (CX4, where X is a halogen atom).
Reaction of Carbon with Oxygen to form Carbon Dioxide:
C(s) + O2(g) → CO2(g)
Reaction of Carbon with Hydrogen to form Methane:
C(s) + 2H2(g) → CH4(g)
Reaction of Carbon with Chlorine to form Carbon Tetrachloride:
C(s) + 2Cl2(g) → CCl4(l)
Reaction of Carbon with Nitrogen to form Cyanide:
C(s) + N2(g) → CN2(g)
Reaction of Carbon with Sulfur to form Carbon Disulfide:
C(s) + 2S(s) → CS2(l)
Reaction of Carbon with Iron to form Iron Carbide:
3Fe(s) + 2C(s) → Fe3C(s)
Reaction of Carbon with Calcium to form Calcium Carbide:
2C(s) + CaO(s) → CaC2(s) + CO(g)
Reaction of Carbon with Water to form Carbon Monoxide:
C(s) + H2O(g) → CO(g) + H2(g)
Carbon Element Diagram:
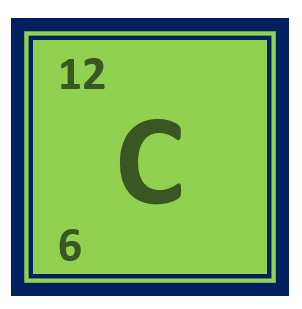
Carbon is a chemical element with the symbol C and atomic number 6. It is a non-metallic element that is found in all living organisms and in many minerals.
Carbon Element Bohr Model:
By <a href=”//commons.wikimedia.org/wiki/User:Alejandro_Porto” title=”User:Alejandro Porto”>Alejandro Porto</a> – <span class=”int-own-work” lang=”en”>Own work</span>, CC BY-SA 3.0, Link
The Bohr model of carbon shows that it has a nucleus containing six protons and six neutrons, surrounded by two energy levels of electrons. The first energy level contains two electrons, while the second energy level contains four electrons.
Carbon Element on Periodic Table:
Carbon is found in group 14 of the periodic table, usually known as the carbon group. It is a non-metal and shares some similarities with other elements in the group, such as silicon, germanium, and tin.
Carbon Tetrachloride:
Carbon tetrachloride, or CCl4, is a colorless, heavy, and dense liquid that is used as a solvent, fire extinguisher, and refrigerant. However, it is also a potent toxic substance and is known to cause serious harm to human health and the environment.
Carbon Monoxide Formula:
Carbon monoxide is a chemical compound with the formula CO. It is a colorless and odorless gas that is produced from incomplete combustion of carbon-containing materials. Carbon monoxide is highly toxic and can cause severe health problems, including death, if inhaled in high concentrations.
Carbon Dioxide Formula:
Carbon dioxide is a chemical compound with the formula CO2. It is a colorless and odorless gas that is produced by the combustion of carbon-containing materials and by the respiration of living organisms. Carbon dioxide is an important greenhouse gas that plays a significant role in global climate change.
Carbon Fiber:
Carbon fiber is a lightweight and strong material that is made from carbon atoms. It is commonly used in aerospace, automotive, and sporting goods industries to make parts that require high strength and low weight.
Carbon Dating:
Carbon dating, sometimes referred to as radiocarbon dating, is a technique used to determine the age of organic materials. It relies on the fact that carbon-14, a radioactive isotope of carbon, decays over time at a known rate.
Carbon Forms:
Carbon can form various forms, including diamond, graphite, and fullerenes. Diamond is a hard and transparent material that is used in jewelry and industrial applications. Graphite is a soft and slippery material that is commonly used in pencils and lubricants. Fullerenes are a group of molecules that have a cage-like structure and are used in medicine, electronics, and nanotechnology.



