What is combustion?
Combustion is a chemical reaction that occurs when a fuel reacts with an oxidizer to produce heat, light, and various by-products. The process of combustion is often referred to as burning.
Combustion typically requires three elements: fuel, oxygen, and an ignition temperature. The fuel is any material that can be burned, such as wood, gasoline, or natural gas. The oxidizer is the source of oxygen that is required for the fuel to burn. The ignition source is a spark or other form of energy that initiates the combustion reaction.
In a controlled combustion process, the fuel and oxidizer are mixed in the right proportions and ignited, resulting in a flame that produces heat and light. In an uncontrolled combustion process, such as a fire, the fuel and oxidizer are not mixed in the right proportions, and the reaction can become explosive or produce harmful by-products, such as smoke.
Combustion is an important process that is used to generate heat and power in many different applications, including heating homes and buildings, cooking food, and generating electricity.
Differences between Complete combustion and incomplete combustion of fuels
Combustion is the chemical process of burning a fuel to release energy in the form of heat and light. Two distinct combustion processes exist: complete combustion and incomplete combustion.
Complete combustion occurs when a fuel is burned in the presence of sufficient oxygen, resulting in the production of carbon dioxide and water as the only byproducts. This process is considered efficient because it releases a significant amount of energy and produces minimal harmful emissions.
Incomplete combustion occurs when a fuel is not burned completely, resulting in the production of carbon monoxide and other harmful byproducts. This process is less efficient because it releases less energy and produces harmful emissions that can have negative effects on the environment and human health.
Here are some key differences between complete combustion and incomplete combustion:
Oxygen supply: Complete combustion requires a sufficient supply of oxygen to support the reaction, while incomplete combustion can occur when there is not enough oxygen present.
Byproducts: Complete combustion produces only carbon dioxide and water as byproducts, while incomplete combustion produces carbon monoxide and other harmful emissions such as particulate matter and unburned hydrocarbons.
Efficiency: Complete combustion is more efficient because it releases more energy and produces fewer harmful emissions, while incomplete combustion is less efficient because it releases less energy and produces more harmful emissions.
Health and environmental impact: Complete combustion has a minimal impact on human health and the environment because it produces only carbon dioxide and water, while incomplete combustion can have negative effects on both due to the production of harmful emissions
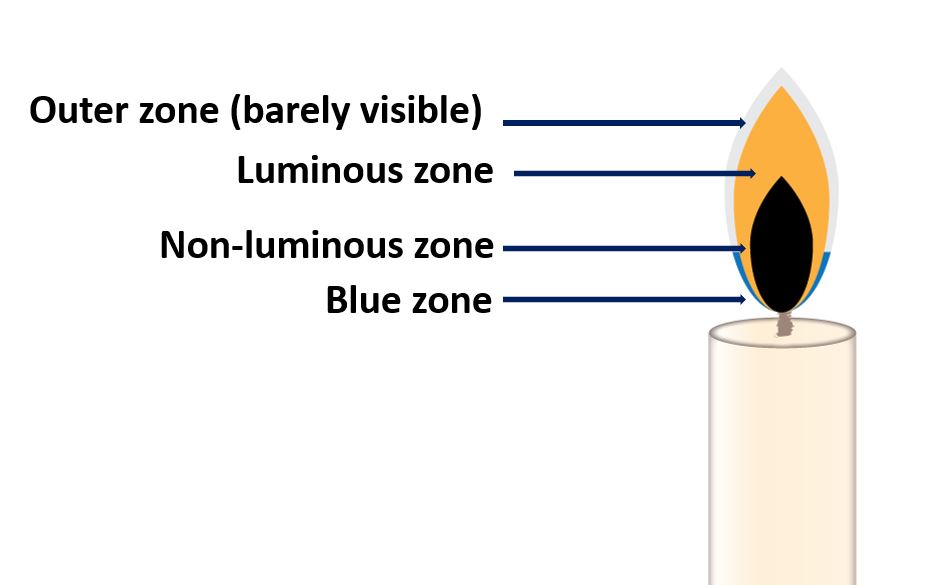
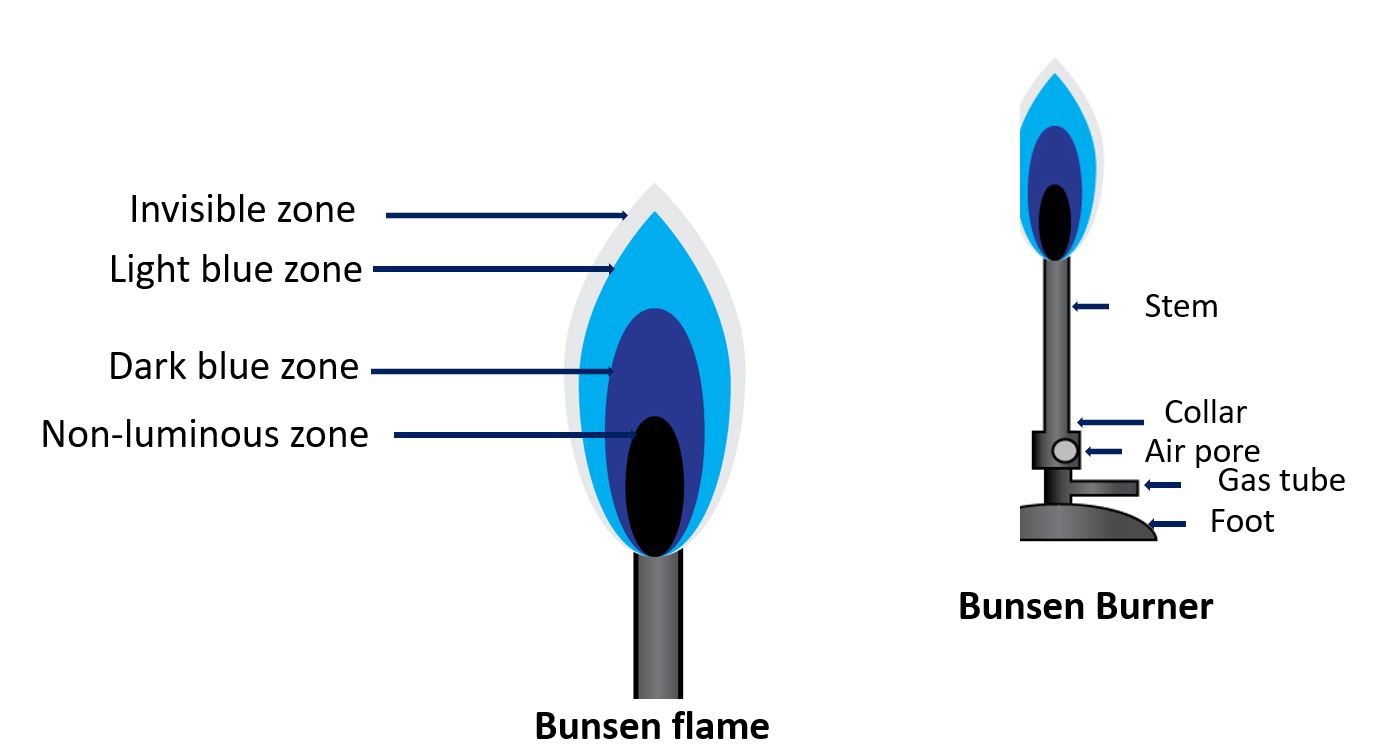
Candle flame vs Bunsen flame
A candle flame is a type of flame that is produced by the burning of a candle. It is typically small and relatively low in temperature, and it is primarily used for lighting or decoration.
A Bunsen flame, on the other hand, is a type of flame that is produced by burning a gas, typically natural gas or propane, through a Bunsen burner. This type of flame is much hotter and more intense than a candle flame, and it is often used in scientific laboratories for heating, sterilization, and other purposes.
One key difference between a candle flame and a Bunsen flame is the amount of heat they produce. A candle flame is relatively cool, with a temperature of around 2250 degrees Fahrenheit, while a Bunsen flame can reach temperatures of up to 2700 degrees Fahrenheit.
Another difference is the size and shape of the flame. A candle flame is usually small and cone-shaped, while a Bunsen flame is typically larger and more diffuse, with a blue center and a yellow outer region.
In summary, a candle flame is a small, low-temperature flame used primarily for lighting and decoration, while a Bunsen flame is a larger, hotter flame used in scientific laboratories for heating and other purposes.
what is ignition temperature?
Ignition temperature is the lowest temperature at which a substance will catch fire and begin to burn. The ignition temperature of a substance depends on several factors, including the chemical composition of the substance, the presence of oxygen, and the surrounding temperature and humidity. For example, the ignition temperature of paper is around 480 degrees Fahrenheit, while the ignition temperature of gasoline is around 536 degrees Fahrenheit. It is important to be aware of the ignition temperature of various substances, as it can help to prevent fires and ensure safe handling of flammable materials.
When a flammable substance is heated to its ignition point, it begins to burn.
As a result, three major factors required for combustion can be determined. They are as follows:
- The presence of a combustible substance
- Access to a combustion promoter (oxygen).
- Heating the combustible material to its ignition temperature.
Fire triangle
The fire triangle is a model that illustrates the three elements that are necessary for a fire to occur: fuel, oxygen, and heat. These three elements are known as the “fire triangle” because they are the three sides of a triangle, with heat at the center. The fire triangle is a useful tool for understanding the basic requirements for a fire and for developing strategies to prevent or control fires.
To start a fire, you need a fuel source, such as wood, paper, or gasoline. The fuel must be able to burn, which means that it must contain enough combustible material to sustain a flame.
In addition to fuel, a fire needs oxygen to burn. Oxygen is present in the air we breathe, and it is necessary for the chemical reaction that occurs when a fuel burns. Without enough oxygen, a fire will not be able to sustain itself.
Finally, a fire needs heat to start and continue burning. The heat can come from a variety of sources, such as a match, a lighter, or a spark. The heat must be high enough to ignite the fuel and sustain the fire.
Understanding the fire triangle is important for fire prevention and fire safety. By removing one of the elements of the fire triangle, you can prevent a fire from starting or extinguish a fire that is already burning. For example, if you remove the heat source, the fire will go out. If you remove the oxygen, the fire will also go out. And if you remove the fuel, the fire will not be able to start in the first place.