Helium Element
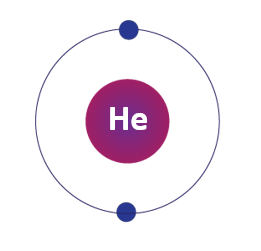
Symbol of helium element
Symbol of helium element is denoted as He.
Helium is a chemically inert noble gas with a number of distinct physical and chemical properties. Here is a summary of helium under various topics.
Physical properties of helium element:
At normal temperature, helium is a colorless, odorless, tasteless, and non-toxic gas.
It is the second-lightest element after hydrogen, with a density about 7 times less than that of air.
Helium has a very low boiling point and is the only element that cannot be solidified by lowering the temperature.
Chemical properties of helium element:
Helium is a noble gas, which means it is generally chemically inert and does not readily form chemical bonds with other elements.
It has a full valence shell with two electrons, which makes it very stable and unreactive.
Helium can form weak van der Waals bonds with other atoms and molecules, but these are very weak interactions.
Helium can form weak van der Waals bonds with other atoms and molecules, but these are very weak interactions.
Valency of helium element:
Helium has a valency of 0, which means it does not readily form chemical bonds with other elements.
It has a full valence shell with two electrons, which makes it very stable and unreactive.
Electronic configuration of helium element:
The electronic configuration of helium is 1s2, which means it has two electrons in the first energy level.
It has a full valence shell, making it highly stable and unreactive.
Uses of helium element:
- Helium is widely used as a cooling agent in cryogenics and in cooling nuclear reactors.
- It is also used in gas chromatography, as a lifting gas for balloons and airships, and in arc welding and cutting.
- Helium is used in medical imaging, such as MRI machines, as a breathing gas for deep-sea diving, and to treat certain lung conditions.
Reactions with other elements:
Helium is generally inert and does not readily form chemical bonds with other elements.
It can form weak van der Waals bonds with other atoms and molecules, but these are very weak interactions.
Industrial uses of helium element:
- Many industrial applications use helium, including welding, electronics, and fiber optics.
- It’s also used to make semiconductors and other electronic components.
Medical uses of helium element:
Helium has medical uses, particularly as a breathing gas for people with respiratory conditions like asthma and chronic obstructive pulmonary disease (COPD).
Periodic table helium element
The chemical element helium is represented on the periodic table by the symbol He and the atomic number 2. Because of its stability and resistance to combining with other elements, helium is classified as a “noble gas”
Helium element picture
A picture of the helium atom typically depicts two small spheres representing the protons and neutrons in the nucleus, surrounded by a cloud of electrons in orbit around the nucleus. The electrons are often shown as small dots or circles around the nucleus, representing their position in the electron cloud.
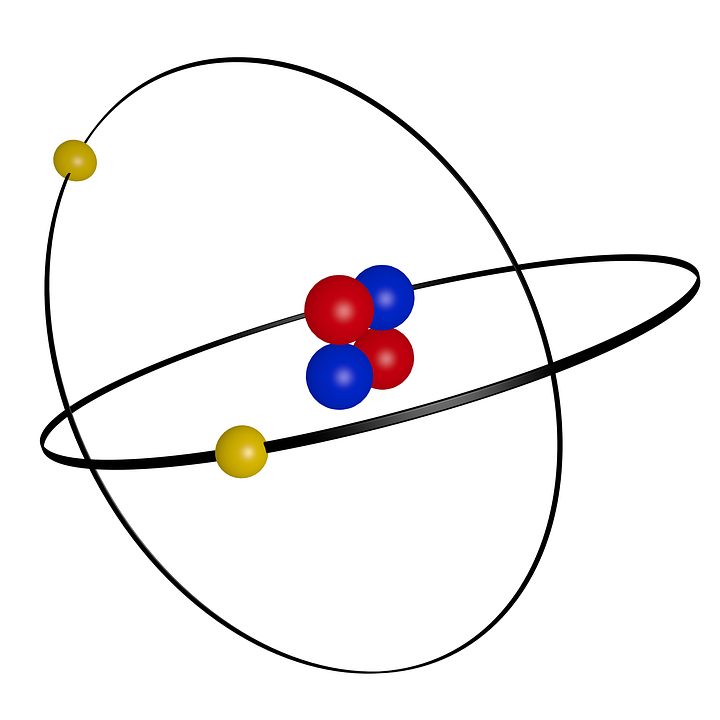
Helium element model
The model of the helium atom is based on the principles of quantum mechanics, which describe the behavior of electrons and other subatomic particles. According to this model, the helium atom has two protons and two neutrons in the nucleus, and two electrons in orbit around the nucleus.
Compound of helium element with 3 atoms
Helium does not typically form compounds with other elements due to its highly stable electronic configuration. However, there is one known compound of helium that contains three atoms of helium, known as He3. He3 is a rare isotope of helium that is formed in nuclear reactions and can be used for various scientific and technological applications.
Helium element protons
The atomic number of helium is 2, which means that it has two protons in the nucleus. Protons are positively charged particles that determine the identity of an element, as the number of protons in the nucleus is unique for each element.
Helium element neutrons
The most common isotope of helium, known as He4, has two neutrons in the nucleus along with the two protons. Neutrons are neutral particles that contribute to the mass of the nucleus but do not affect the element’s chemical properties.
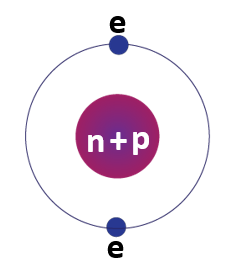
Helium element bohr model
The Bohr model of the helium atom is a simplified representation of the electronic structure of helium that was proposed by Danish physicist Niels Bohr in 1913. According to this model, the two electrons in the helium atom are in the first energy level, and they are held in place by the attraction between their negatively charged electrons and the positively charged nucleus. The Bohr model is useful for understanding the basic properties of atoms, but it has been largely superseded by more complex models based on quantum mechanics.