Lithium is a soft, silvery-white metal that is highly reactive and has a number of unique physical and chemical properties.
Lithium symbol: Li
Is lithium a metal?
Yes, lithium is a metal. It is a soft, silver-white metal that is highly reactive and flammable. Lithium is the lightest metal and the solid element with the highest specific heat capacity.
Physical properties of Lithium element:
Lithium is a soft, silvery-white metal with a low density and low melting and boiling points.
It is the least dense solid element and the metal with the least weight.
Lithium has a low heat conductivity and is a good electrical conductor.
Chemical properties of Lithium element:
Lithium is a highly reactive metal that readily forms compounds with a wide range of other elements and compounds.
It strongly reacts with water, releasing hydrogen gas and a solution of lithium hydroxide.
Lithium can form compounds with both nonmetals and metals, and can exist in various oxidation states.
Valency of Lithium element:
Lithium has a valency of +1, which means it tends to lose one electron to form a stable cation in chemical reactions.
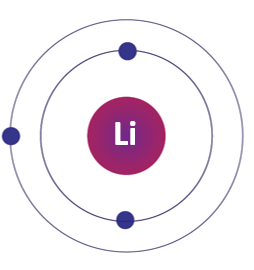
Lithium electron configuration:
The electronic configuration of lithium is 1s2 2s1, which means it has two electrons in the first energy level and one electron in the second energy level.
What is lithium used for?
Uses of Lithium element:
Lithium is used in the production of rechargeable batteries, particularly those used in portable electronic devices like laptops and smartphones.
It is also used to make ceramics and glass, as well as a heat transfer agent in nuclear reactors.
Reactions of Lithium element with other elements:
When lithium reacts with water, it produces hydrogen gas and a solution of lithium hydroxide.
Equation for reaction of lithium with water
Lithium + Water → Lithium hydroxide + Hydrogen gas
Balanced Equation for reaction of lithium with water
2Li + 2H2O → 2LiOH + H2
It also reacts with many other elements, including halogens, oxygen, and nitrogen, to form a range of compounds.
Equation for reaction of lithium with Oxygen
Lithium + Oxygen → Lithium oxide
Balanced Equation for reaction of lithium with Oxygen
4Li + O2 → 2Li2O
Equation for reaction of lithium with Chlorine gas
Lithium + Chlorine gas → Lithium chloride
Balanced Equation for reaction of lithium with Chlorine gas
2Li + Cl2 → 2LiCl
Equation for reaction of lithium with Fluorine gas
Lithium + Fluorine gas → Lithium fluoride
Balanced Equation for reaction of lithium with Fluorine gas
2Li + F2 → 2LiF
Equation for reaction of lithium with Nitrogen gas
Lithium + Nitrogen gas → Lithium nitride
Balanced Equation for reaction of lithium with Nitrogen gas
6Li + N2 → 2Li3N
Lithium iodide
Lithium iodide formula
Lithium iodide is an inorganic compound with the chemical formula LiI. It is a white, crystalline solid that is soluble in water and has a high melting point.
Uses of Lithium iodide
Lithium iodide is commonly used as a source of iodine for pharmaceuticals and as a catalyst in organic chemistry.
Lithium chloride
Lithium chloride formula
Lithium chloride is a white, crystalline compound with the chemical formula LiCl. It is soluble in water and has a high melting point.
Uses of Lithium chloride
Lithium chloride is used in a variety of applications, including as a desiccant, in the production of ceramics, and as a flux for soldering and welding.
Lithium hydroxide
Lithium hydroxide is a white, crystalline compound. It is soluble in water and has a high melting point.
Lithium hydroxide formula
The chemical formula for lithium hydroxide is LiOH.
Uses of Lithium hydroxide
Lithium hydroxide is commonly used in the production of lithium-ion batteries, as well as in the production of ceramics and glass.
Lithium oxide
Lithium oxide formula
Lithium oxide is an inorganic compound with the chemical formula Li2O. It is a white, crystalline solid that is insoluble in water and has a high melting point.
Uses of Lithium oxide
Lithium oxide is used as a flux in the production of ceramics and glass, as well as in the production of lithium-ion batteries.
Lithium bromide
Lithium bromide formula
Lithium bromide is an inorganic compound with the chemical formula LiBr. It is a white, crystalline solid that is soluble in water and has a high melting point.
Uses of Lithium bromide
Lithium bromide is commonly used as a desiccant and as a coolant in air conditioning systems.
Lithium sulfate
Lithium sulfate formula
Lithium sulfate is an inorganic compound and its chemical formula is Li2SO4. It is a white, crystalline solid that is soluble in water and has a high melting point.
Uses of Lithium sulfate
Lithium sulfate is used in the production of lithium-ion batteries, as well as in the production of glass and ceramics.
Lithium nitride
Lithium nitride formula
Lithium nitride is an inorganic compound with the chemical formula Li3N. It is a yellow, crystalline solid that is insoluble in water and has a high melting point.
Uses of Lithium nitride
Lithium nitride is used in the production of ceramics and as a catalyst in organic chemistry.
Lithium carbonate
Lithium carbonate formula
The chemical formula for lithium carbonate is Li2CO3. It is a white, crystalline compound.
Uses of Lithium carbonate
It is an inorganic salt that is used in a variety of industrial and medical applications. Lithium carbonate is produced from lithium-containing brines or minerals such as spodumene or petalite. It is a key ingredient in the production of lithium-ion batteries, which are used to power electronic devices such as laptops, smartphones, and electric cars.
Lithium Bohr Model:
Lithium batteries
Lithium batteries are a type of rechargeable battery that uses lithium as its primary component. These batteries are widely used in portable electronic devices such as laptops, smartphones, and digital cameras due to their high energy density and long lifespan. Lithium batteries have become increasingly popular in recent years, with advancements in technology leading to improved performance and decreased cost.
How to dispose of lithium batteries
Lithium batteries should be disposed of properly to prevent environmental damage. Many communities have battery recycling programs that accept lithium batteries. If a recycling program is not available, lithium batteries can be disposed of in the trash, but they should be placed in a sealed plastic bag to prevent fires caused by short-circuiting.
Lithium battery recycling
Lithium battery recycling is the process of recovering materials from used lithium batteries. The recycling process involves breaking down the batteries and separating the various components, including lithium, cobalt, nickel, and copper. These materials can then be reused in the production of new batteries and other products.
How is lithium mined
Lithium is typically mined from brine.