Matter and how it is classified
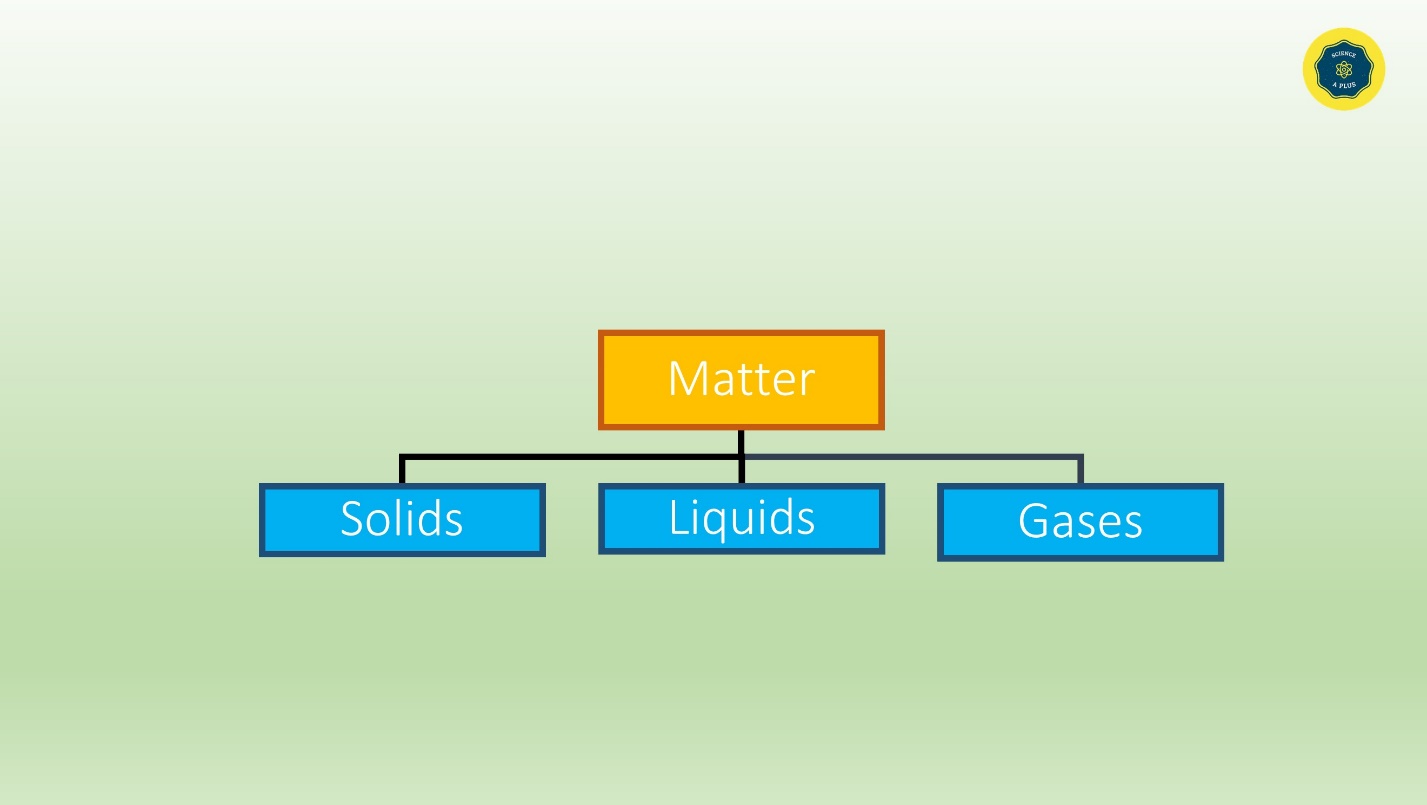
Everything that surrounds us is matter. Matter is solids, liquids and gases.
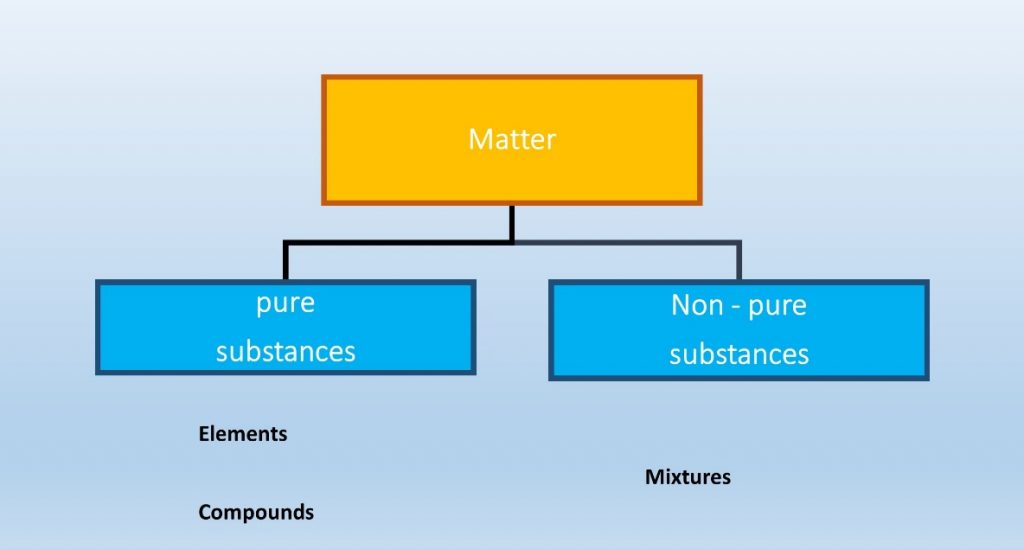
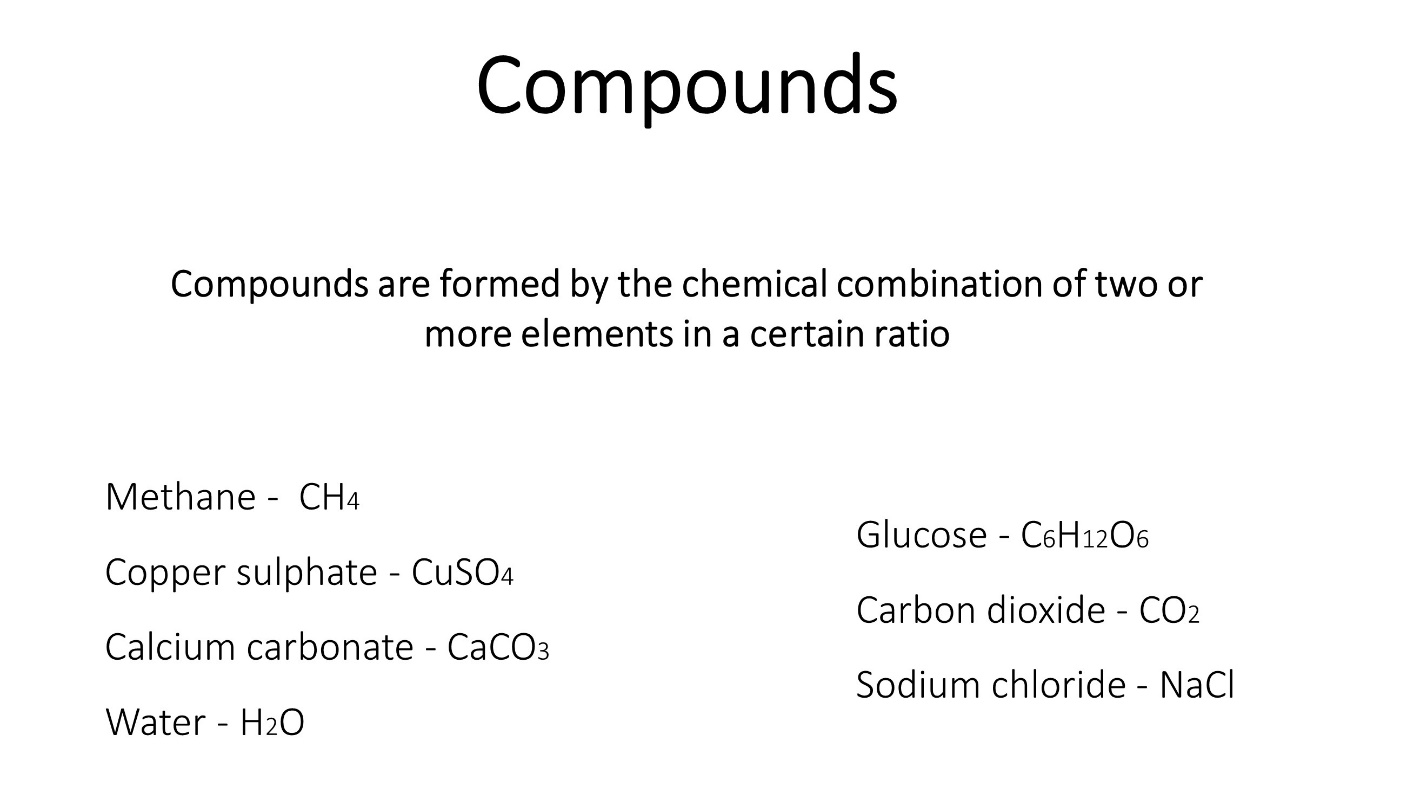
Matter can be divided as pure and non-pure.
Elements and compounds are pure substances of matter and mixtures are non-pure substances of matter.
Building unit of matter is the Atom
Atoms build matter. Atom cannot be divided further. The structure of the atom was described by several scientists.
A Brief history of atomic theory
In chronological order, the first model of the atom was proposed by the ancient Greeks. The Greeks envisioned that all matter was composed of four basic elements (fire, earth, air, and water), and they believed that these elements were made up of tiny particles called atoms.
In 1803 John Dalton proposed an atomic theory based on the law of conservation of mass. Dalton theorized that atoms are indestructible and indivisible; they cannot be created or destroyed but can be combined in various ways to form different compounds. He also said that compounds are formed when atoms are joined together in simple whole-number ratios.
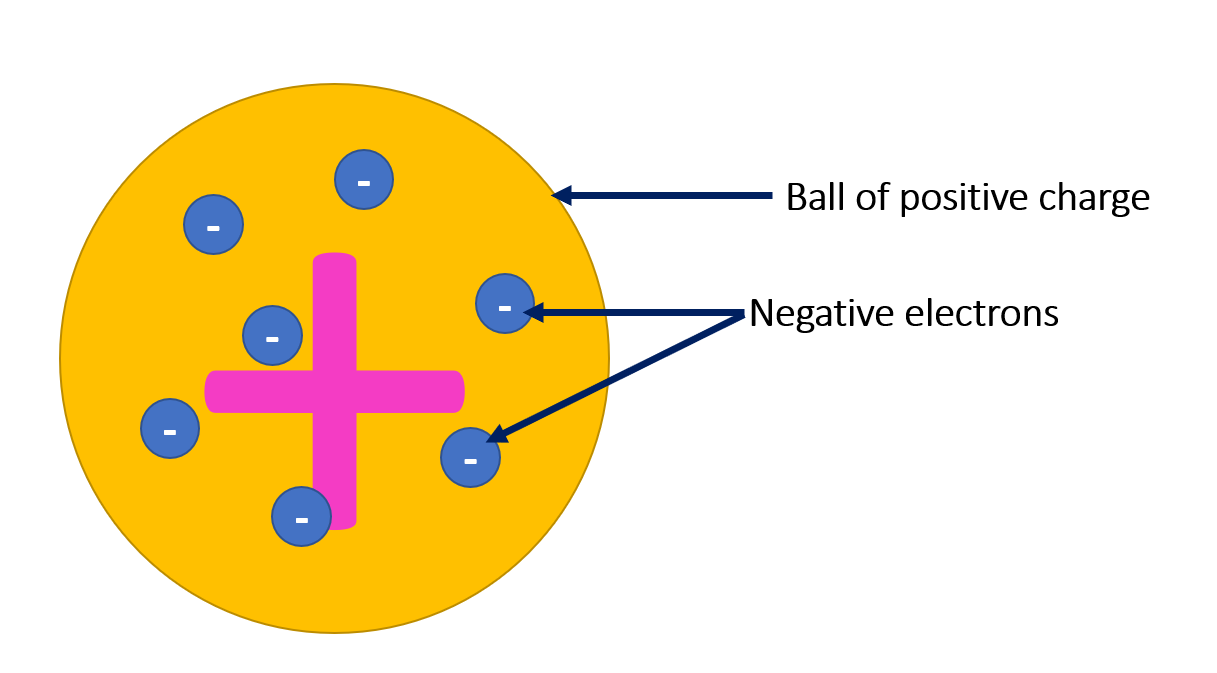
Prior to Rutherford’s model, the prevailing theory of atomic structure was the plum pudding model, proposed by English physicist J.J. Thomson in 1904. Thomson had discovered and identified the electron, which he described as a fundamental negatively charged particle. He postulated that it was uniformly embedded within a positively charged sphere, to balance out its charge. According to this view, atoms were shapeless clouds of positive matter with electrons scattered throughout them like plums in a pudding.
Rutherford’s experiments on alpha particles (He+ nuclei) propelled from radioactive sources were critical in developing the modern view of the atom: In 1909 he showed that a small fraction of alpha particles was deflected at large angles when they collided with thin gold foil; such large-angle scattering indicated that most of the mass of an atom is concentrated in a region much smaller than previously thought possible. Rutherford suggested that there was a dense core within atoms, which he called a nucleus, surrounded by lighter electrons. The nucleus contained most or all of an atom’s positive charge and nearly all of its mass.
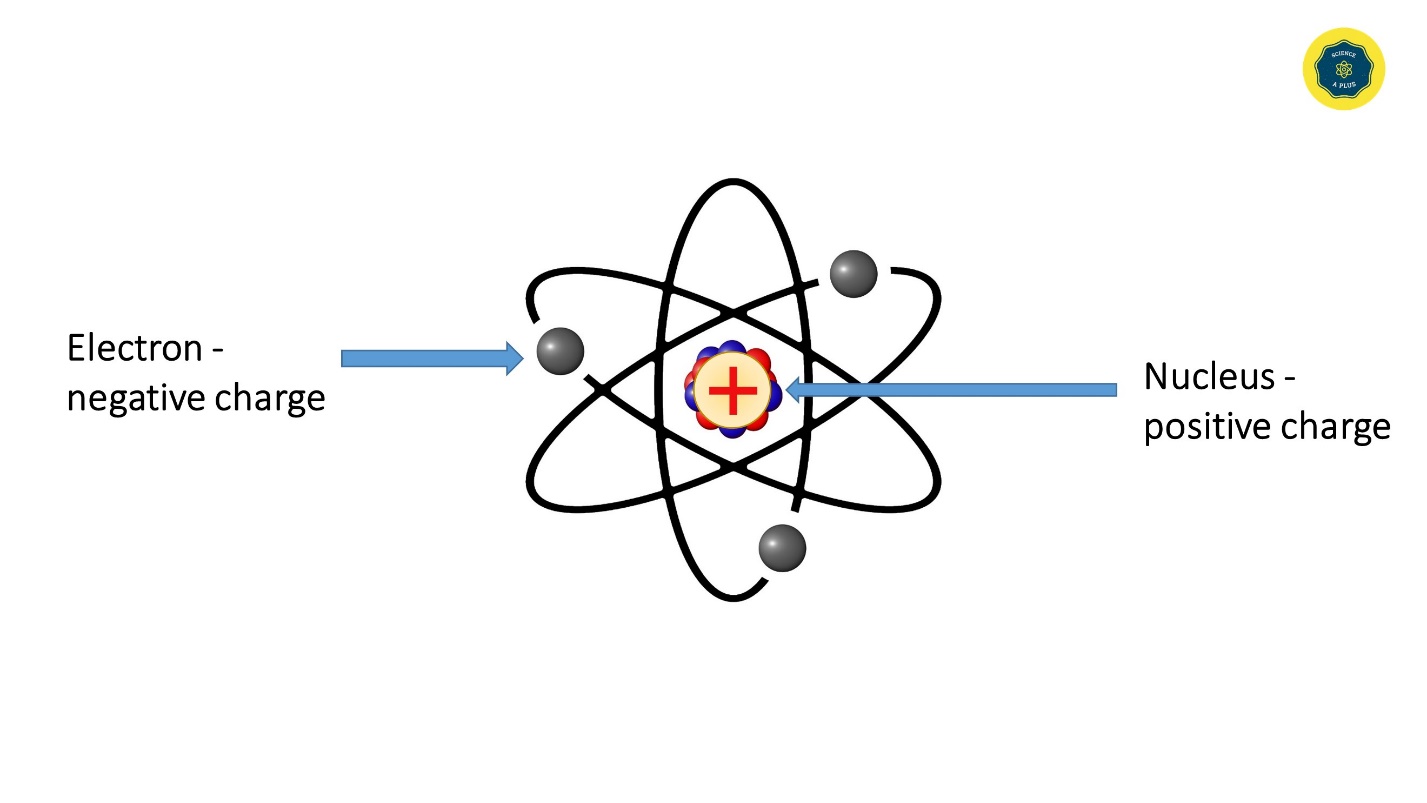
The Planetary Model of the Atom
Rutherford concluded that atoms must be mostly empty space and that an atom’s mass is concentrated in a very tiny, positively charged nucleus; negative electrons are dispersed around it like planets orbiting the Sun. The fact that some alpha particles were deflected only slightly indicated that most of them passed through empty space between atoms; but those that bounced back had encountered dense positively charged material in the nucleus of an atom. Thus, Rutherford’s model provided a clear picture of how positive and negative charges are distributed in an atom and explained why atoms are electrically neutral.
The planetary model of the atom was introduced by Ernest Rutherford (1911).
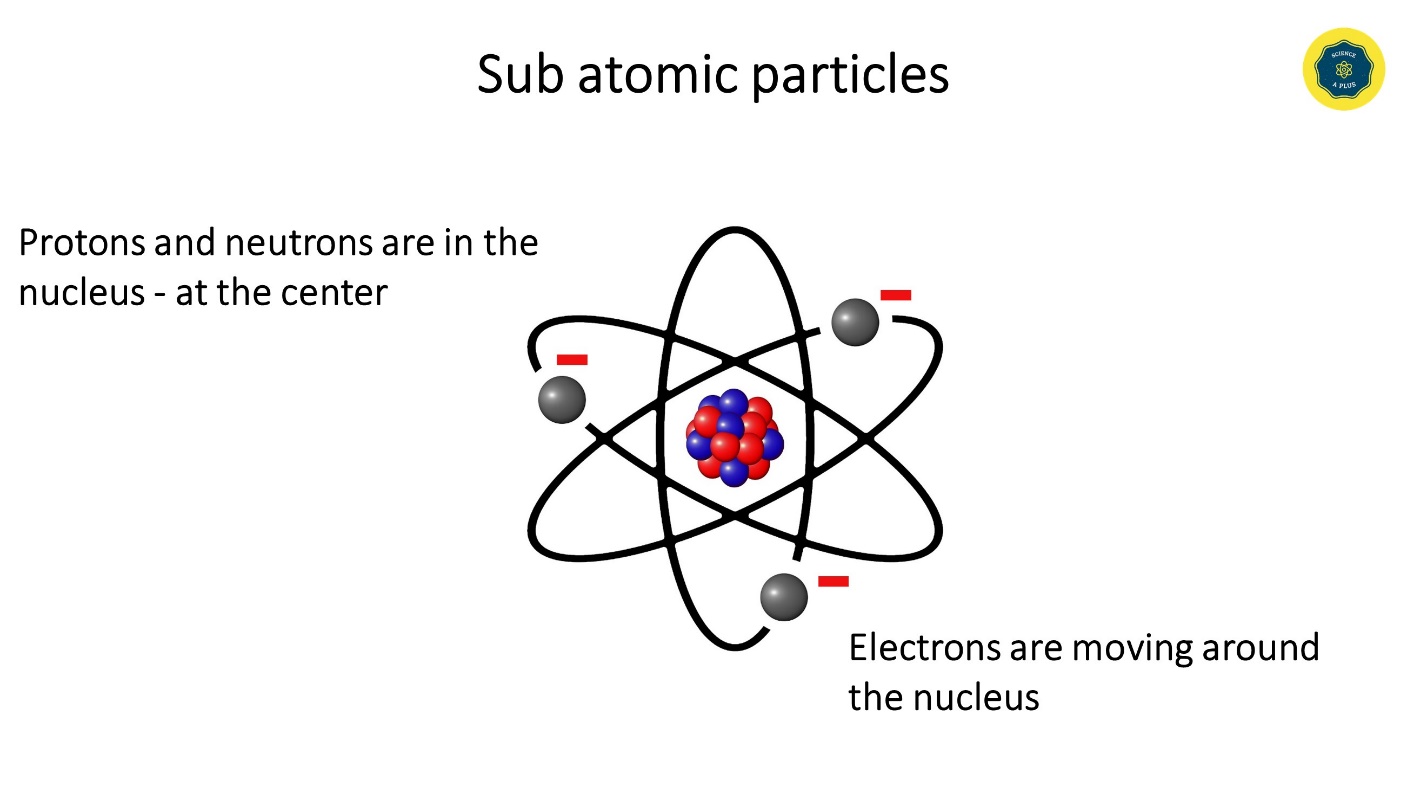
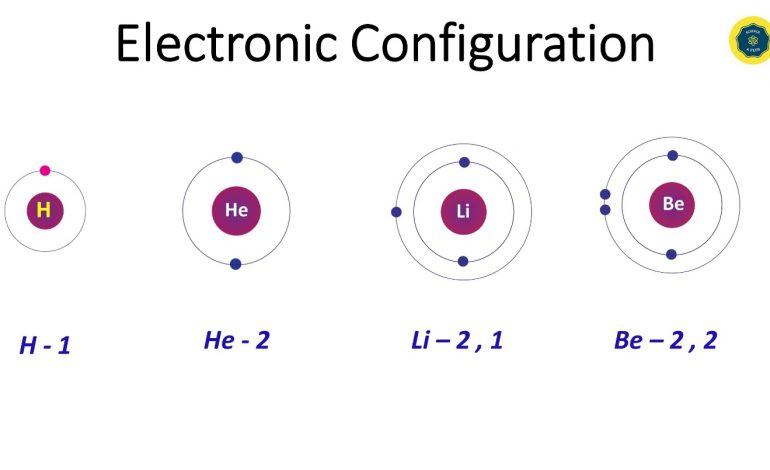
The positive charge of the nucleus attracts electrons. Electrons revolve very fast around the nucleus so that they do not fall on the nucleus.
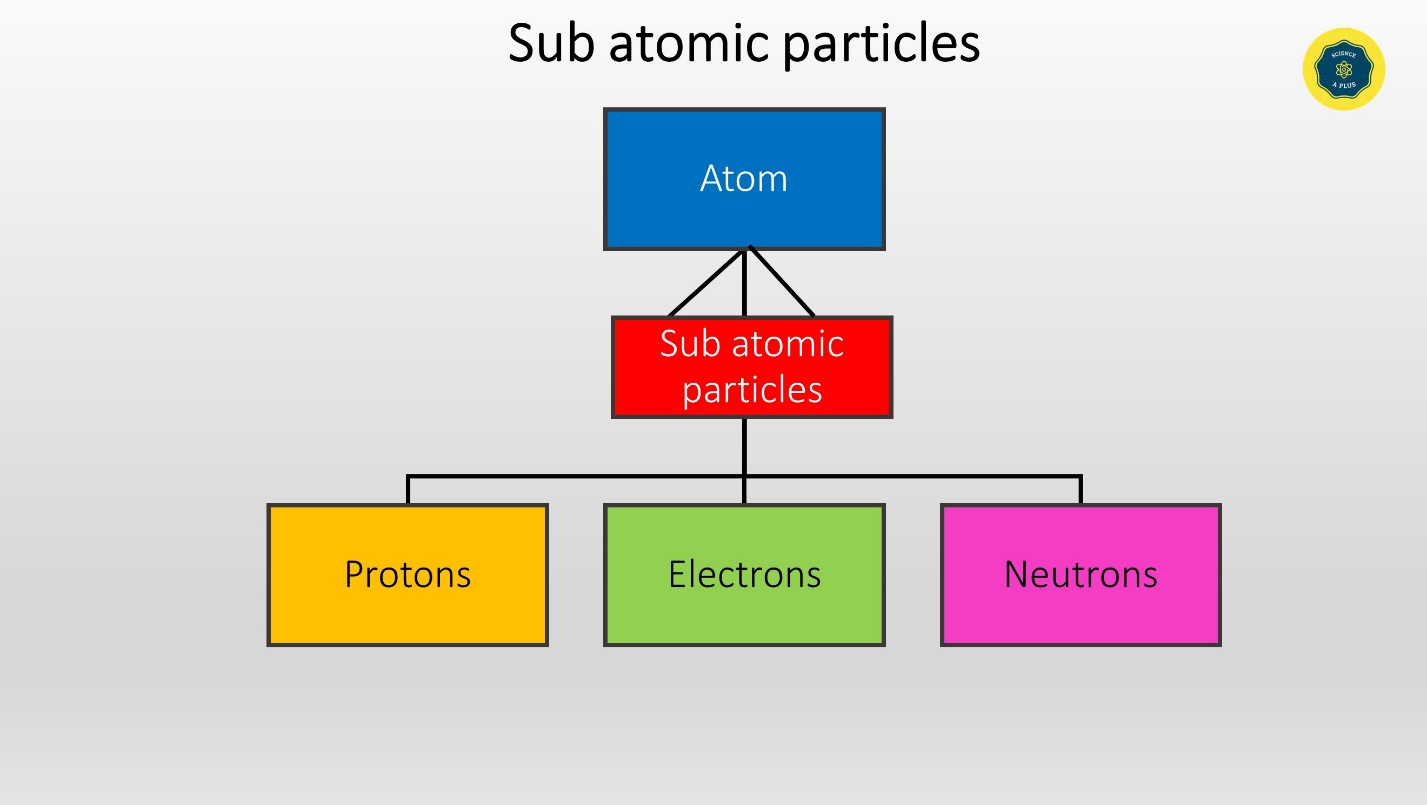
Sub atomic particles
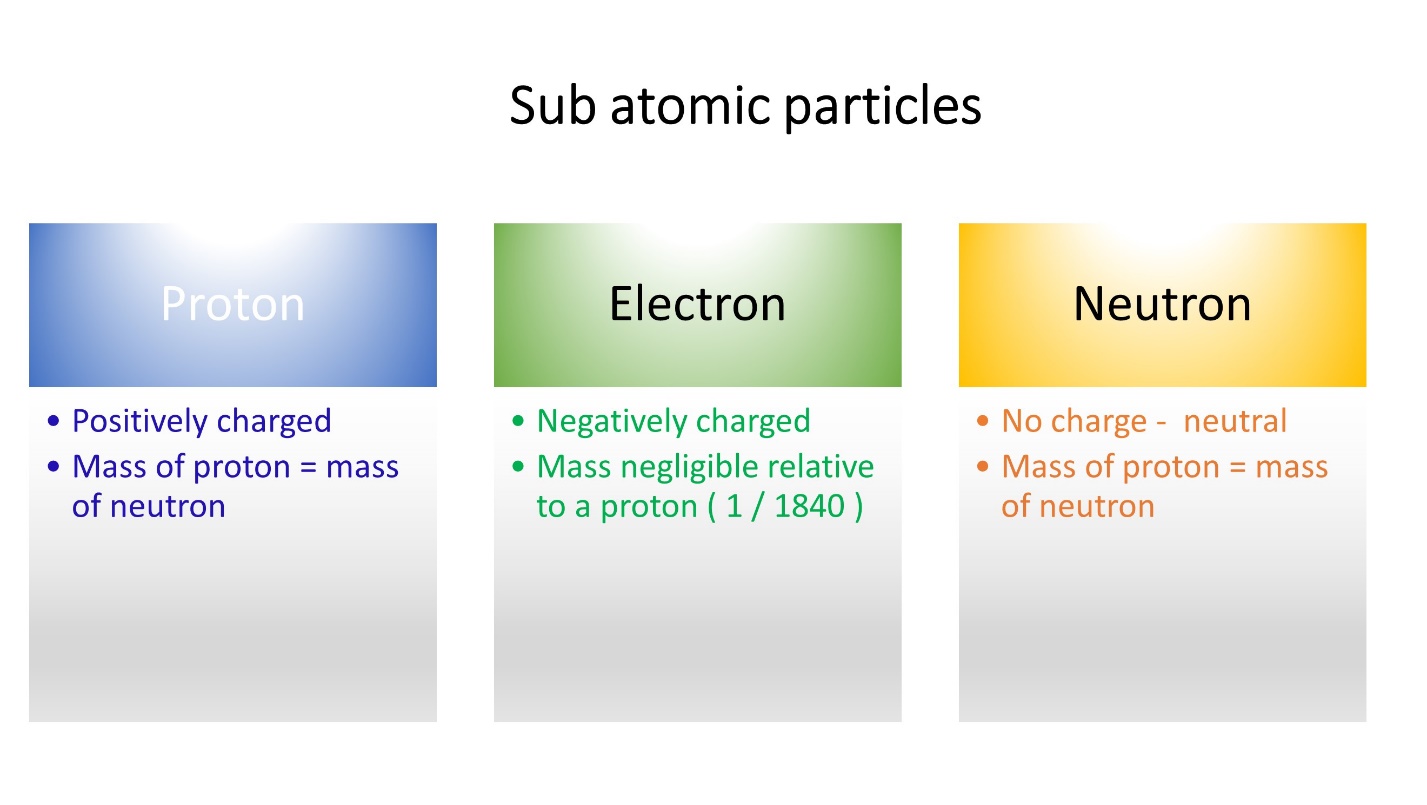
Protons, Neutrons and Electrons are the sub atomic particles in the atom.
These sub atomic particles can be found either in the center, in the nucleus or in the surrounding.
Protons and neutrons gather in the nucleus whereas electrons move around the nucleus.
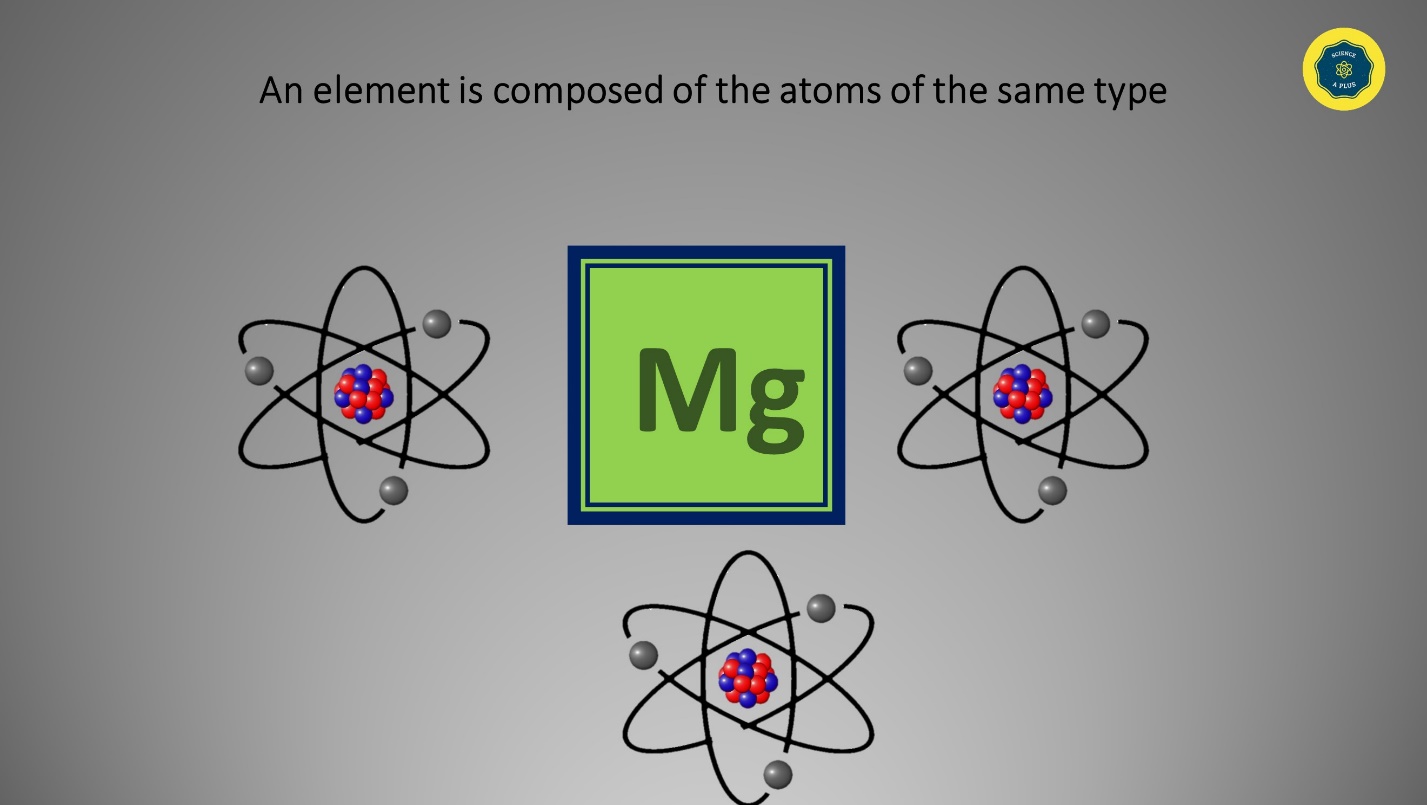
Elements and Compounds
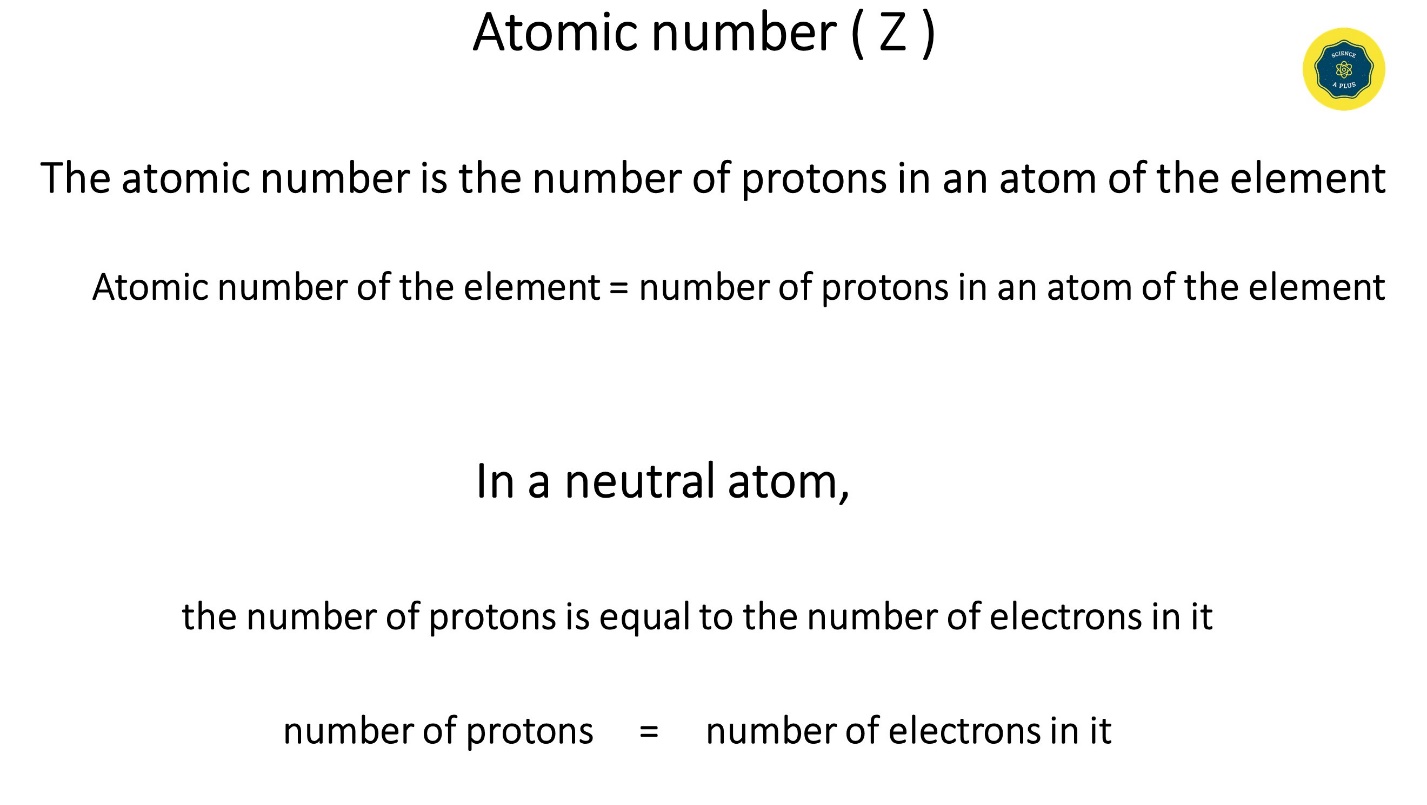
Atomic number ( Z )
- The atomic number is the number of protons in an element’s atom.
- Atomic number of the element = number of protons in an atom of the element
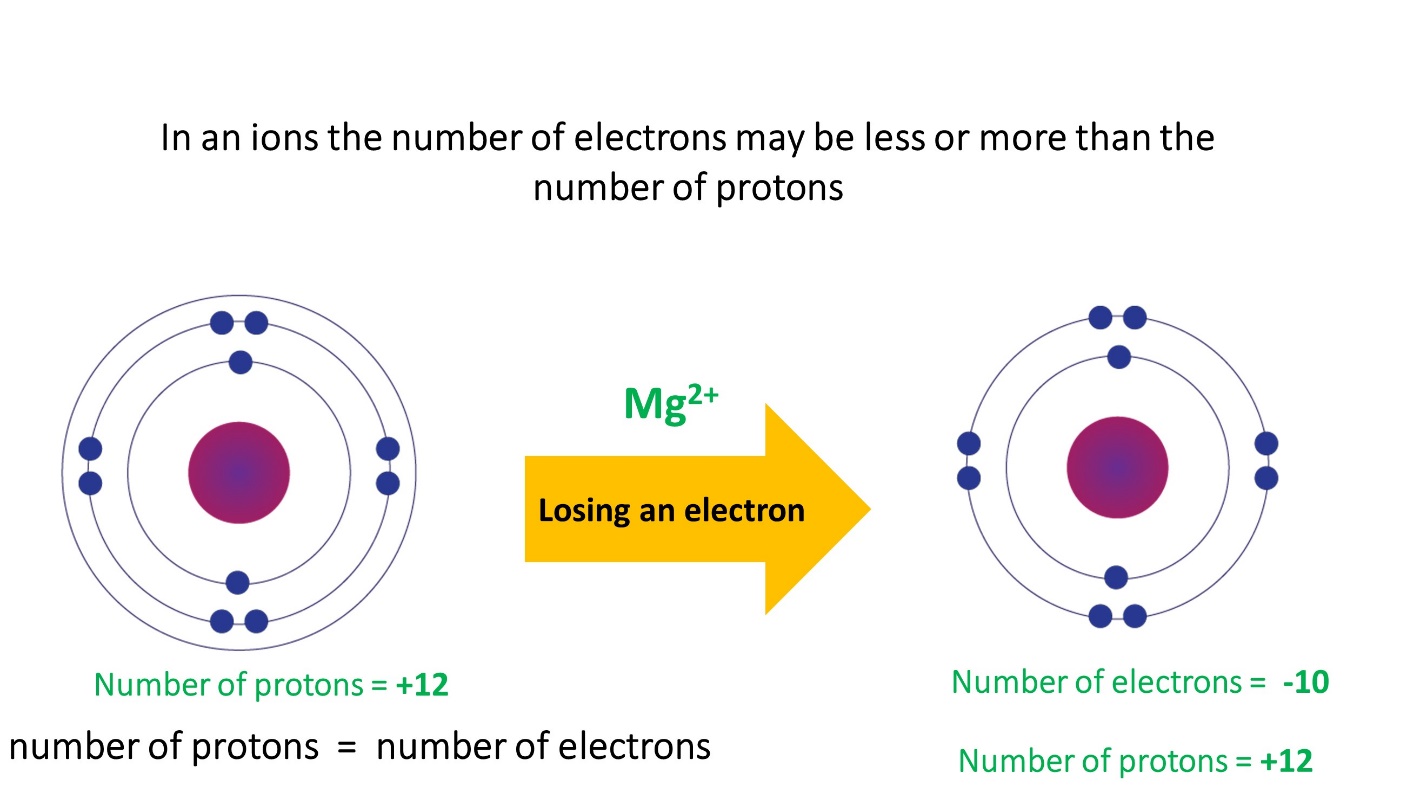
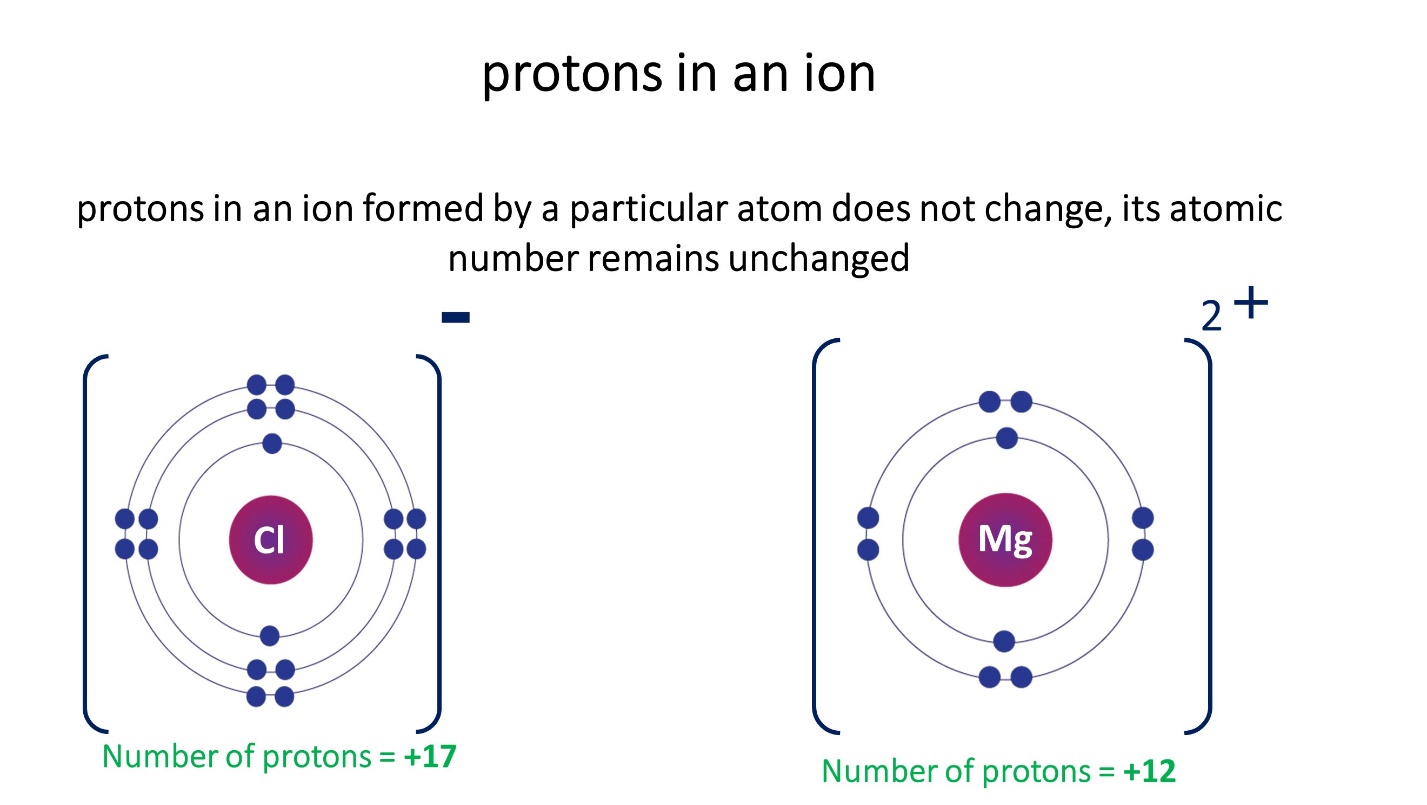
- In cations the number of electrons is less than the number of protons
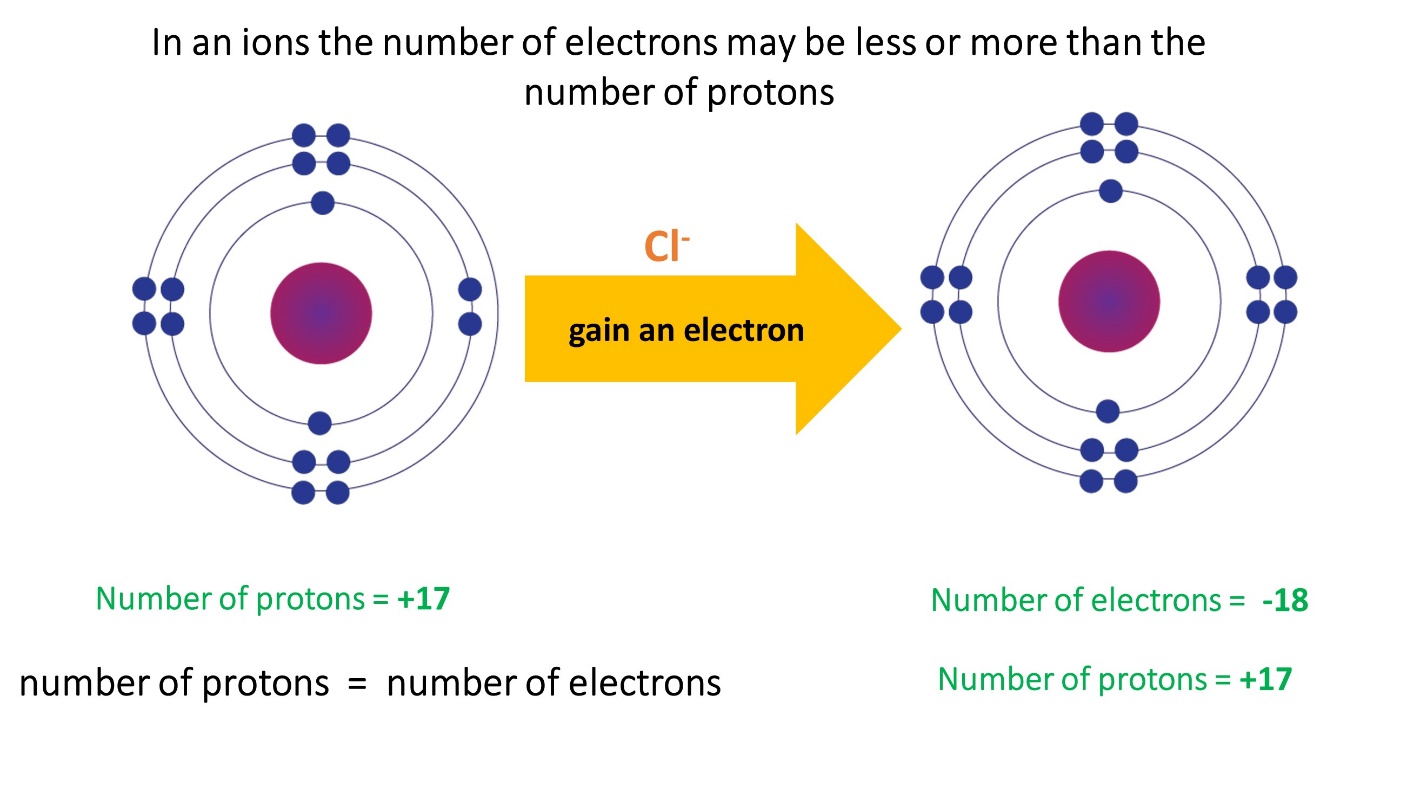
- The number of electrons in anions is greater than the number of protons.
Note that the number of protons in an ion formed by a particular atom does not change, its atomic number remains unchanged
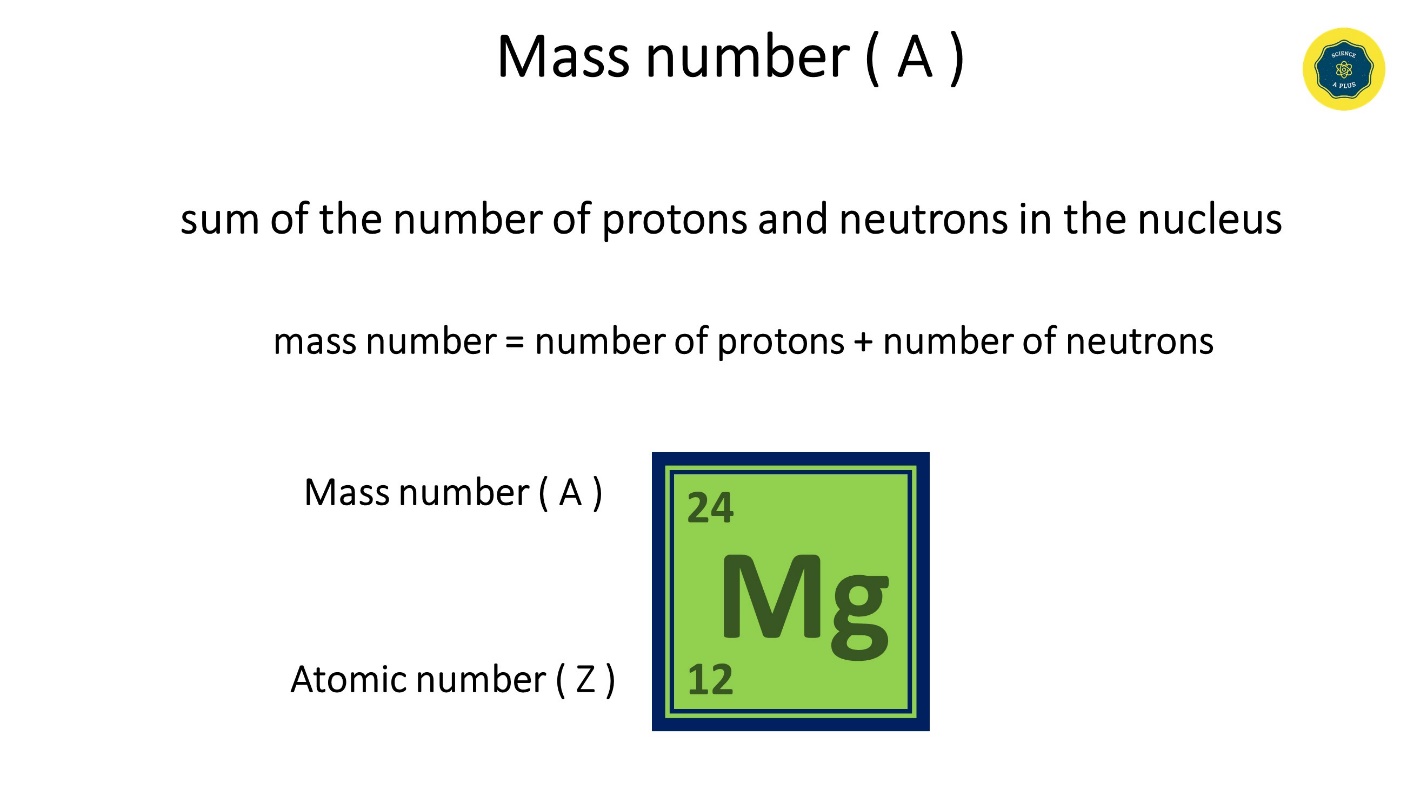
Mass number ( A )
- It is the sum of the number of protons and neutrons in the nucleus
- mass number = protons + neutrons. The mass number is equal to the sum of the number of protons and neutrons.
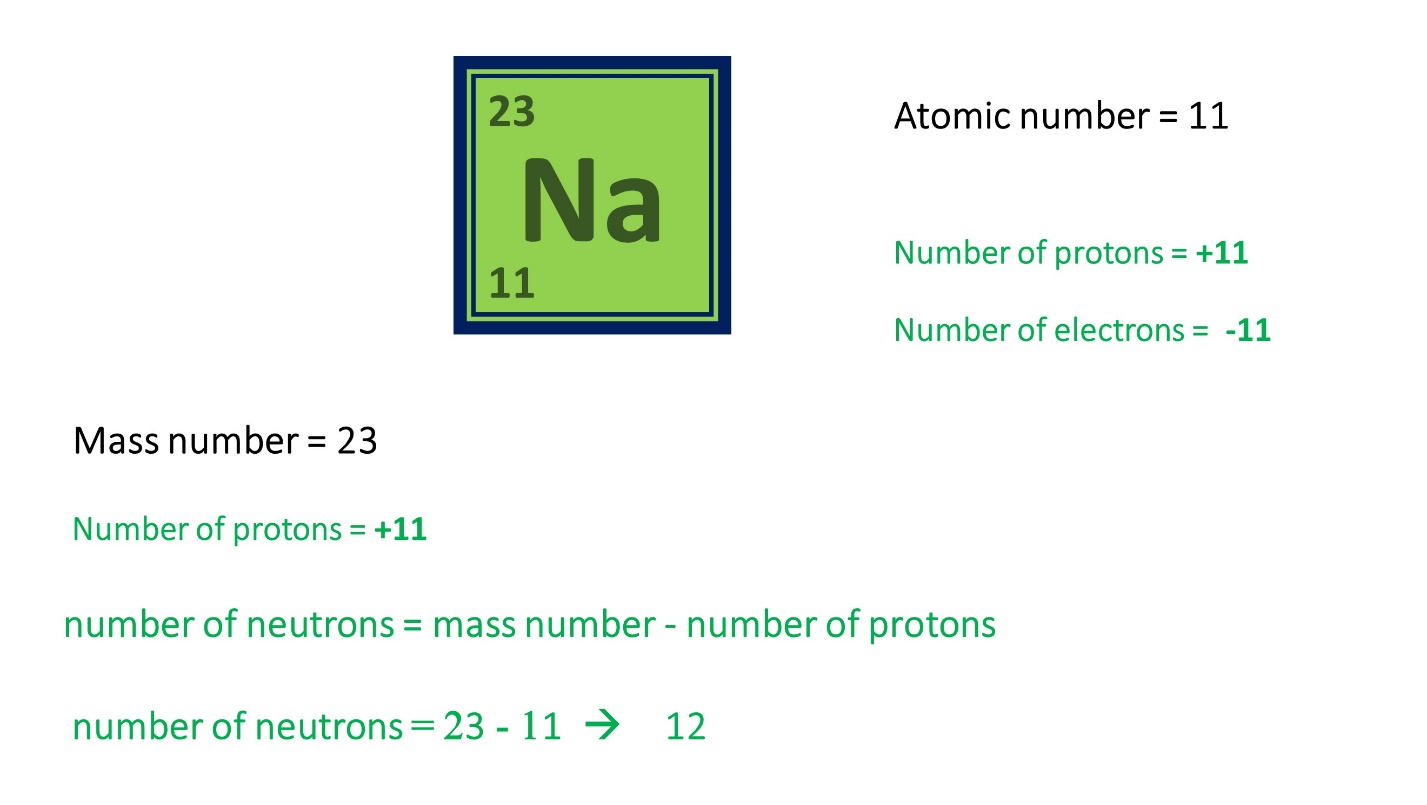
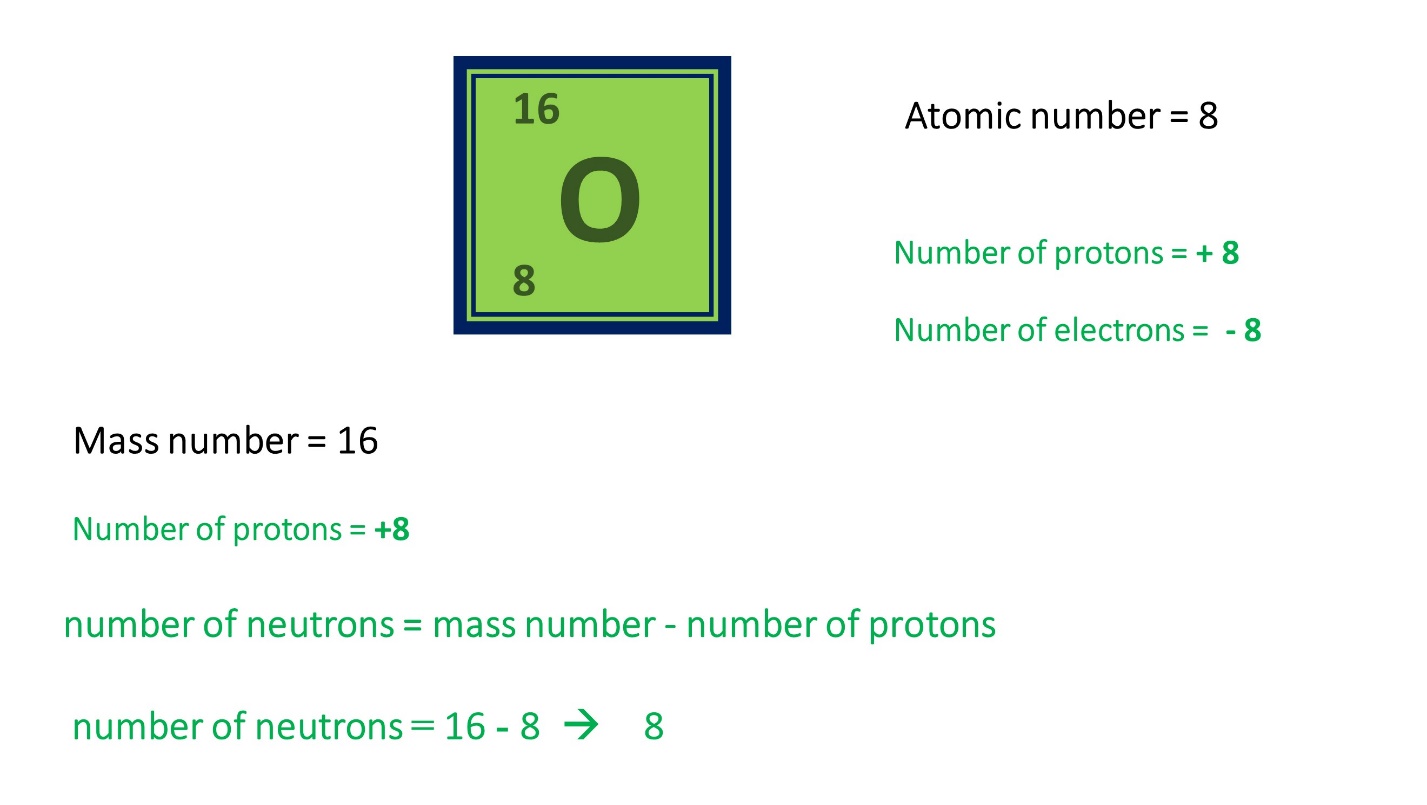
Here are some examples to clarify this theory lesson.