Gases – Their Preparation, Properties and Uses
Hydrogen (H2)
Hydrogen accounts for a tiny fraction of the atmosphere’s total chemical makeup. It has the lowest density of any gas.
- The most common and lightest element in the universe is hydrogen gas.
- It is highly flammable and can burn or explode if it comes into contact with a spark or flame.
- It is often used as a fuel for rockets and as a coolant in nuclear power plants.
- It can be produced from a variety of sources, including natural gas, coal, and water.
- Pure hydrogen gas is odorless, colorless, and tasteless, but it is often mixed with other gases to give it a detectable odor.
Physical and chemical properties of hydrogen gas
Hydrogen has a lower density than normal air. It has a relative molecular mass of two. It is a flammable gas. In water, hydrogen is only mildly soluble. It has no color. The gas has no odor.
Preparation of Hydrogen gas in the laboratory
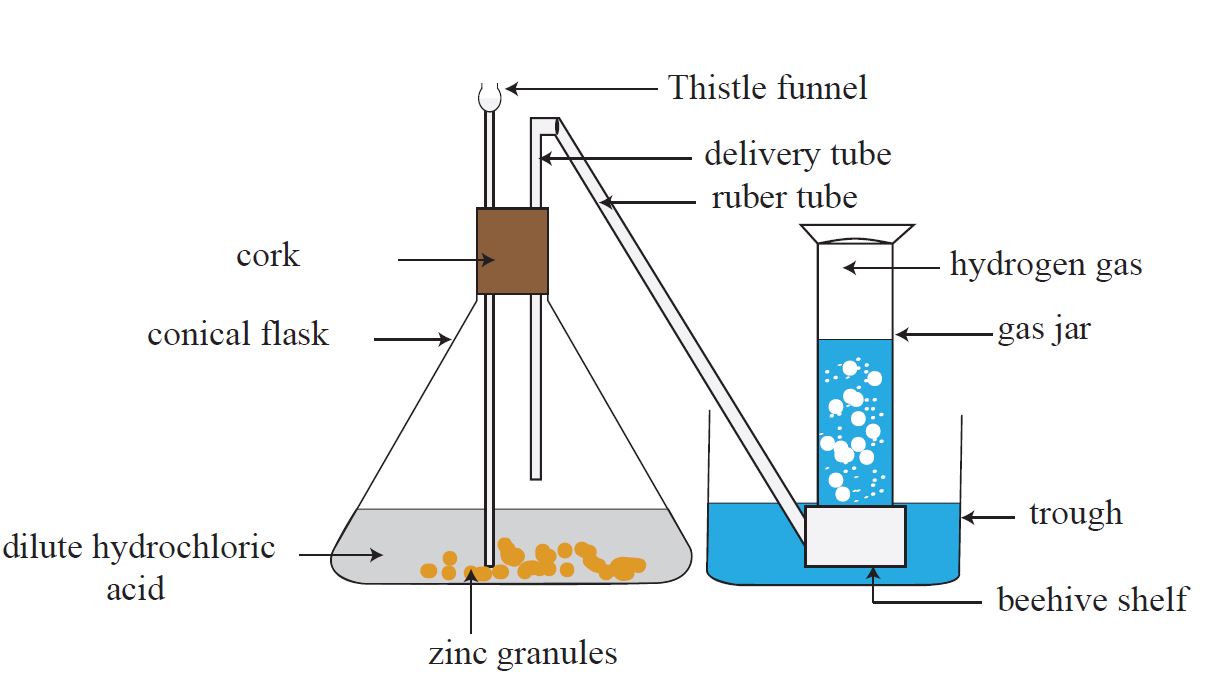
In the lab, hydrogen gas can be produced by the reaction of a metal such as zinc or magnesium with an acid such as dilute hydrochloric acid or dilute sulphuric acid. For example, zinc or magnesium can be used.
Zn + 2 HCl 🡪 ZnCl2 + H2
The apparatus shown below can be used to collect hydrogen gas produced by a reaction like the one described above.
Because the water in the gas jar is pushed down as the gas enters it, this method of collecting a gas is known as downward displacement of water.
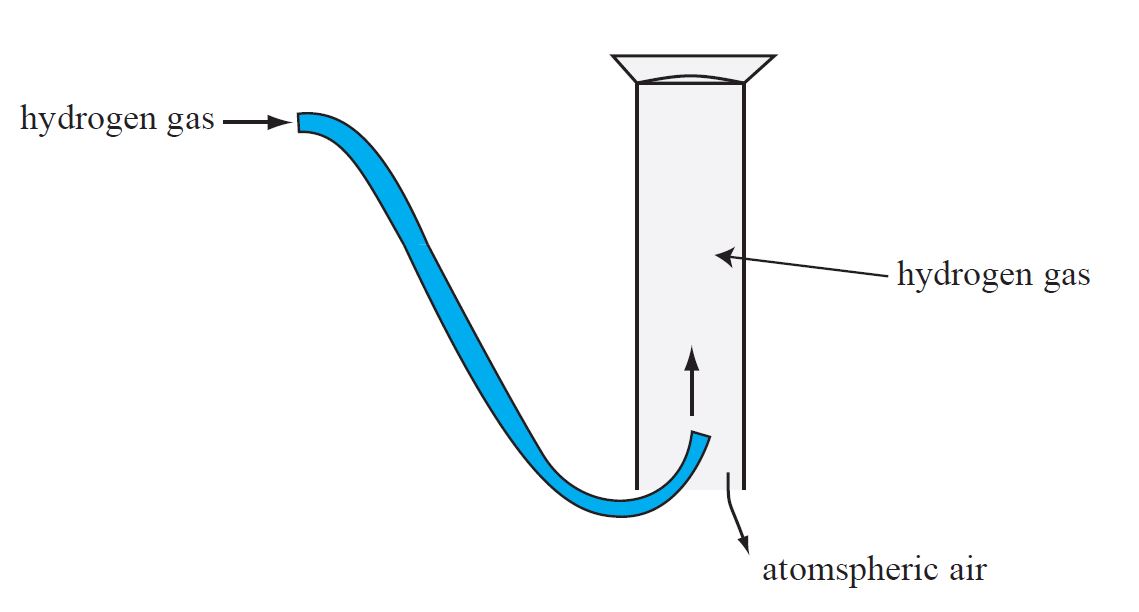
A gas jar, as shown in Figure, can also be used to collect gas from the delivery tube.
Because hydrogen gas has a lower density than normal air, it rises in the atmosphere.
The atmospheric air in the jar is then forced down and extracted. The term ‘downward displacement of air’ refers to this method of collecting a gas.
How to identify hydrogen gas?
When inserting a lit ekel into the gas tube With a squeaky ‘pop,’ the gas burns. This allows hydrogen gas to be easily detected.
Uses of hydrogen gas
Utilized in the art of rocketry as a fuel. Producing margarine out of vegetable oils is an industry. Ammonia gas is produced through the reaction of nitrogen and other elements (Ammonia is used to produce fertilizers such as urea.). lowering of the levels of organic molecules.
Preparation of oxygen gas (O2) in the laboratory
The chemical element oxygen gas has the symbol O and the atomic number 8.
It is the third most abundant element in the universe, accounting for around 21% of the Earth’s atmosphere.
Oxygen is necessary for the survival of most living organisms, as it is required for the process of respiration.
It is a highly reactive element and is involved in many chemical reactions, including the burning of fossil fuels and the corrosion of metals.
Pure oxygen gas is a pale blue gas with a strong, identifiable odor. At room temperature and standard pressure, it is a gas.
Physical and chemical properties of oxygen
The density is significantly higher compared to that of normal air.
The relative molecular mass is equivalent to 32. A proponent of the action of starting fires. Water solubility is only marginally affected. A gas that has no odor and no color.
The following is a list of reactions that can be carried out in the laboratory in order to produce oxygen.
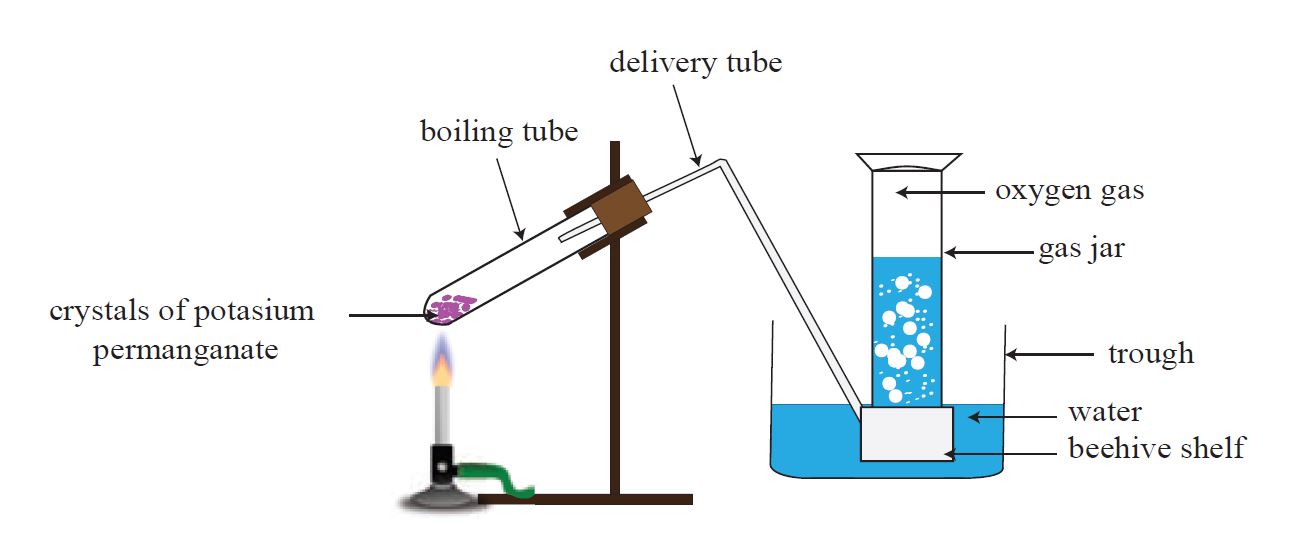
1. Heating potassium permanganate (KMnO4)
2KMnO4 🡪 K2MnO4 + MnO2 + O2
2. Heating potassium nitrate (KNO3)
2KNO3 🡪 2KNO2 + O2
3. Decomposition of hydrogen peroxide (H2O2)
2H2O2 🡪 2H2O + O2
4. Heating potassium chlorate (KClO3)
2KClO3 🡪 2KCl + 3O2
Heat potassium permanganate in the laboratory using the apparatus shown in Figure to produce oxygen gas.
The downward displacement of water is used to gather oxygen gas in this case.
How to identify oxygen gas?
Fill a couple test tubes with oxygen gas. Remove one test tube containing oxygen from the water and insert the glowing splint as soon as the tube’s mouth is opened.
The splint is observed to relight with a flame. This oxygen gas can be recognized.
Applications of oxygen gas
It is necessary for the respiratory processes of every living thing. When something burns, the substance involved reacts with the oxygen in the air. As a result, oxygen is necessary for the process of combustion. It is also utilized in the process of space travel and underwater diving. It is put to work in the process of generating the oxy-acetylene flame that is put to use in the welding of metals. Oxygen is utilized as a primary input in a variety of industrial processes, including the manufacturing of sulfuric and nitric acids.
Preparation of carbon dioxide gas (CO2) in the laboratory
Carbon dioxide is a gas that played a role in the formation of life on Earth. This gas raised the temperature of the Earth’s atmosphere to an appropriate level for living species, and it also serves as a raw material for photosynthesis, the process by which all living beings obtain their food. Carbon dioxide occurs in the atmosphere at a rate as low as 0.03% by volume.
Physical properties of carbon dioxide gas
• It is a gas with a higher density than normal air and a relative molecular mass of 44.
• It does not combustion and does not burn.
• The gas is only marginally soluble in water.
• The gas has no color.
• Carbon dioxide has no odor.
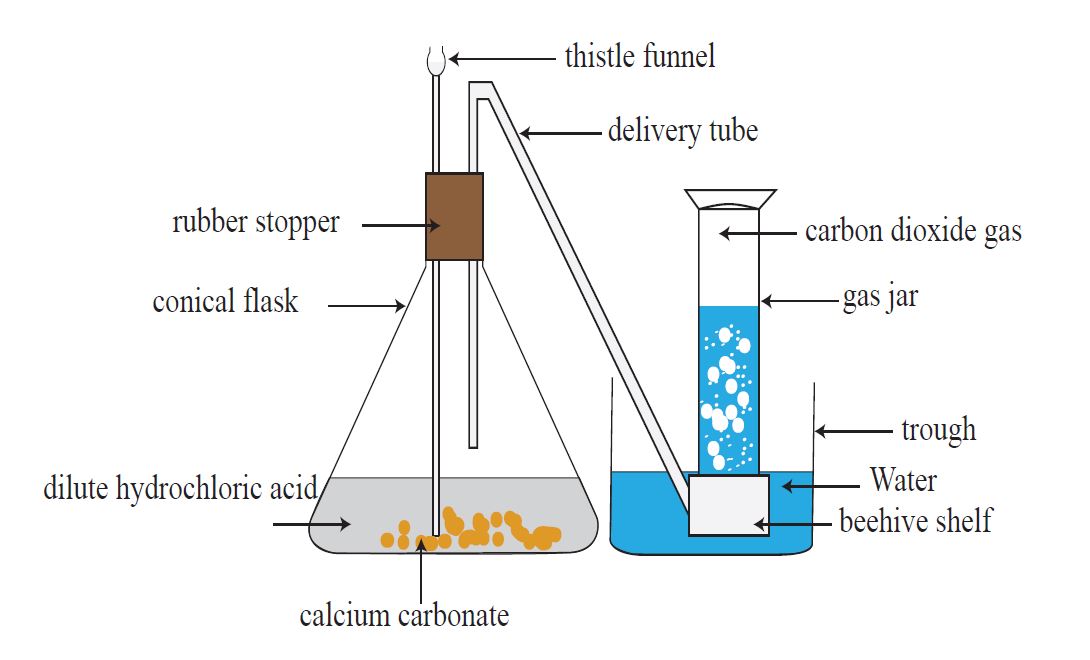
Calcium carbonate (CaCO3) can be converted into carbon dioxide gas by reacting it with weak hydrochloric acid.
CaCO3 + 2HCl 🡪 CaCl2+ H2O + CO2
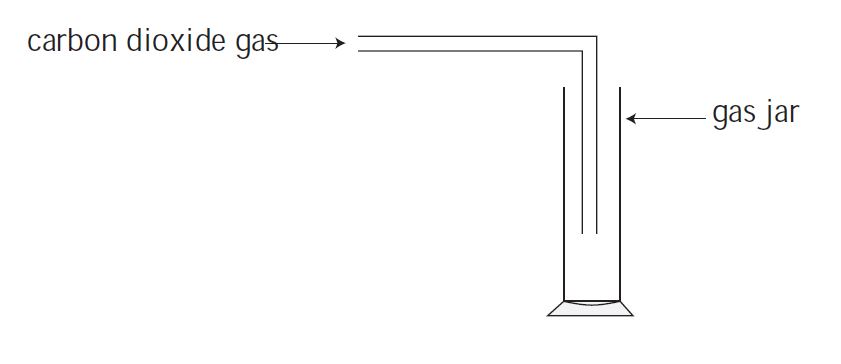
Using the apparatus that is shown in Figure, a sample of carbon dioxide gas can be produced.
To your attention: Although a little quantity of carbon dioxide dissolves in water when collected over water, this is not a barrier to collecting gas samples.
The downward displacement of water is used to gather the gas here as well. However, because carbon dioxide has a higher density than normal air, it can also be collected as shown in Figure.
The carbon dioxide gas from the delivery tube reaches the bottom of the jar due to its high density. The air inside the jar is forced higher when the gas fills it.
As a result, this method of collecting a gas is termed as ‘upward displacement of air’.
How to identify carbon dioxide gas?
Fill a test tube with carbon dioxide with lime water solution, close the tube tightly, and shake vigorously. Fill a test tube halfway with lime water, close it, and shake vigorously. Compare the colors of the two test tubes’ solutions.
In the test tube containing carbon dioxide, lime water becomes more milky. In the test tube, calcium hydroxide in lime water reacts with carbon dioxide as follows.
Ca (OH)2 + CO2 🡪 CaCO3 + H2O
Because the white calcium carbonate generated is suspended in water, the lime water turns milky.
When more carbon dioxide is added into the test tube containing the gas suspension, the gas combines with the calcium carbonate to generate water soluble calcium hydrogen carbonate or calcium bicarbonate [(Ca(HCO3)2. As a result, the solution’s milkiness is reduced.
When carbon dioxide is strongly frozen under high pressure, it solidifies. When heated, solid carbon dioxide converts directly to gas without liquefying, therefore it does not turn into a liquid like ice. As a result, solid carbon dioxide is referred to as dry ice. Dry ice is utilized as a super coolant since its temperature (-770C) is substantially lower than that of ice. Food preservation makes extensive use of dry ice. It is also used to generate artificial rain.
Uses of carbon dioxide
Carbon dioxide is used to extinguish fires because it does not help them burn.
Since carbonic acid (H2CO3), which is made when carbon dioxide dissolves in water, has a taste, it is used to make soda water and other drinks that have a fizz. Reacting carbon dioxide with coke makes carbon monoxide gas, which is needed to get iron extracted from the ground.