Introduction
Elements that are found on the Earth are classified as metals, non-metals, and metalloids. Out of 118 elements in the periodic table over 75% are metals. There are common properties that can be seen in metals. Each metal has its unique features as well. Metals react with other chemicals according to their capability of reacting. Here, we consider the metal reactions with air, water, and acids. Some commonly studied examples are elaborated in this article.
You have a lot of experience in using metals in your day-to-day life for different activities. Have you ever wondered about the behavior of metals with other elements in nature? For example, you might see most iron materials are rusted around the coastal areas.
Let’s see another example. You might have seen gold jewelry does not diminish in color even after a long period of time. Why does that happen? Is there a scientific basis for this phenomenon?
This can be explained with the knowledge of metal reactions.
Metals differ in their readiness of reacting with other materials. Let’s understand it through the knowledge with metal reactions.
Let’s see how different metals react with air, water, and acids in distinct ways.
Reactions of metals with air
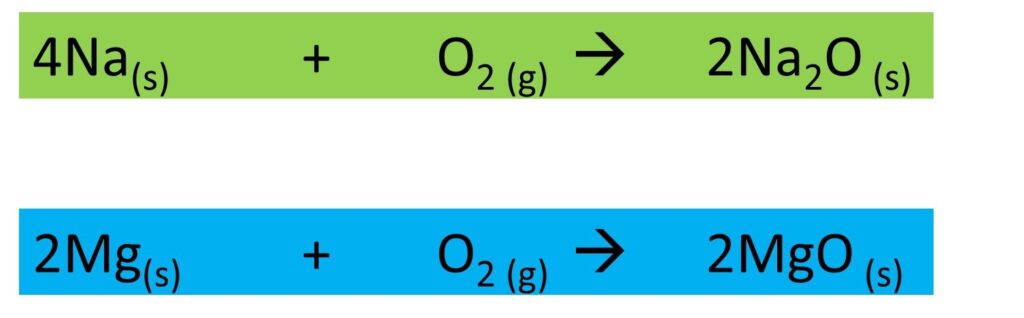
- Sodium metal is a soft metal and it reacts with air fast. When a piece of sodium is exposed to air, its lustrous nature is reduced quickly. The reason for the diminishing color of the surface of sodium metal is its fast reactivity with air. When sodium burns in the air it forms its oxide.
- Magnesium does not react fast with air as much as sodium. It forms its oxide layer around the metal surface.
Here are the chemically balanced equations for the above-mentioned reactions.
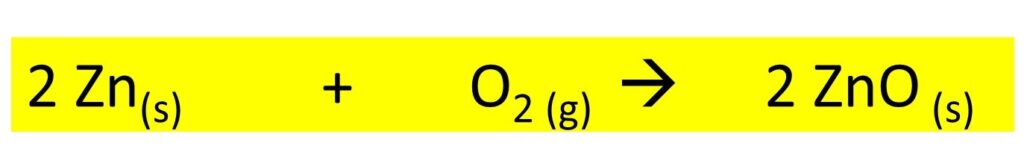
- When the metals like zinc, iron, copper get heated in the air, they become dull in color. These reactions also form a layer of oxide but these reactions need prolonged time and heat.
- Some of the metals like silver, platinum, and gold do not form their oxide even after a long period of exposure.
Reactions of the metals with water
Different metals react with cold water, hot water, and water vapor at different speeds.
- Sodium metal reacts with water violently. When a piece of sodium metal is put into water, it reacts swiftly with a hissing sound. It is more dangerous to put a large particle of sodium metal into water. It catches fire and causes an explosion.
During the reaction of sodium with cold water, it forms its hydroxide and liberates hydrogen gas. Sodium also reacts with hot water and steam vigorously. Therefore, it is dangerous to demonstrate these reactions without safety precautions.
- Magnesium does not react with cold water like sodium. It forms its hydroxide and liberates hydrogen gas when it reacts with hot water.
- Magnesium also reacts fast with steam and produces its oxide and gives hydrogen gas.
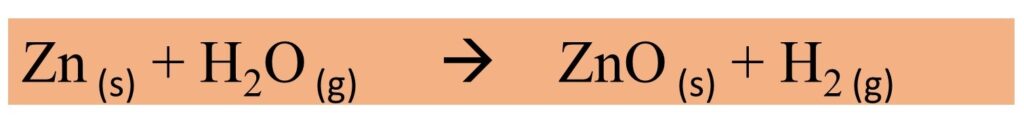
- Metals like aluminium, iron and zinc do not react with cold water or hot water but they react only with steam. They form their oxides and hydrogen gas reacting with steam.
- Some of the metals such as gold, platinum, silver do not react with cold water, hot water, or even with steam.
The reaction of the metals with acids
There are some acids that are used in the laboratory in their dilute form as well as concentrated form. Sulphuric acid, nitric acid, and hydrochloric acid are frequently used in laboratory experiments.
Metals react with these acids at different speeds.
- Highly reactive metals like sodium, potassium, calcium react violently even with dilute acids. Therefore, safety precautions should be taken when doing experiments with these chemicals.
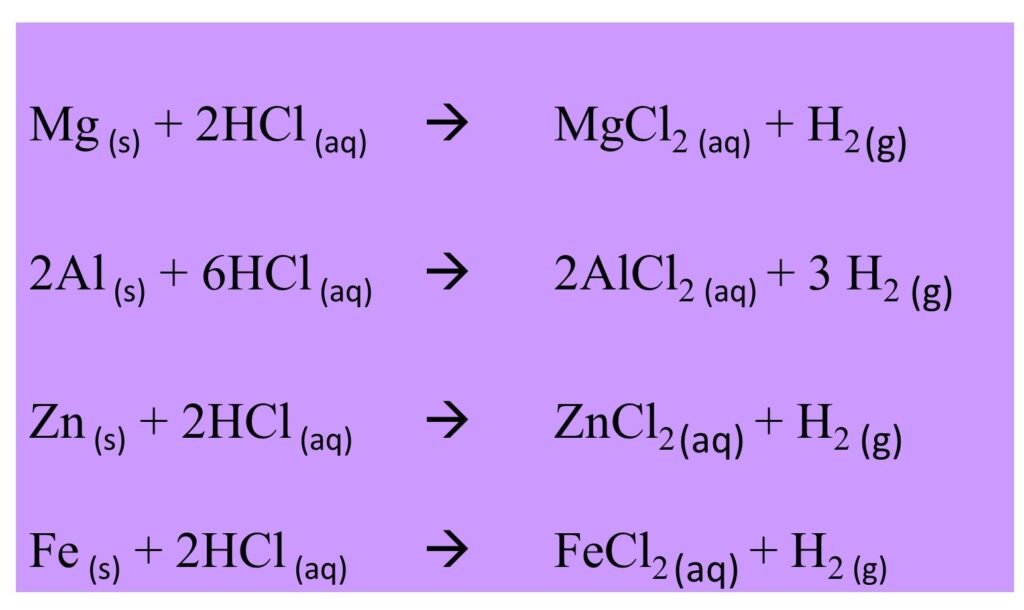
Most metals react with dilute acid at different speeds. Following metals show their reactivity with acids and their speed slows down from the top to the bottom.
Metals with diluted hydrochloric acid
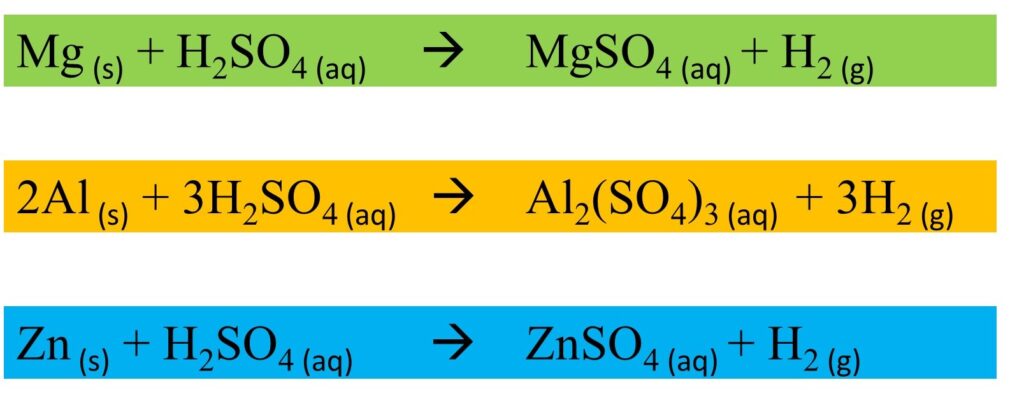
Metals with diluted Sulphuric acid
- The reactivity of metals with acids varies according to the type of acid and its concentration.
For example, copper does not react with dilute hydrochloric acid or dilute sulphuric acid but reacts with concentrated sulphuric acid and forms its sulphate and hydrogen gas.
The reactivity speed of those metals can be predicted by using activity series. It is arranged with the descending order of their reactivity.