What is Vulcanization of rubber
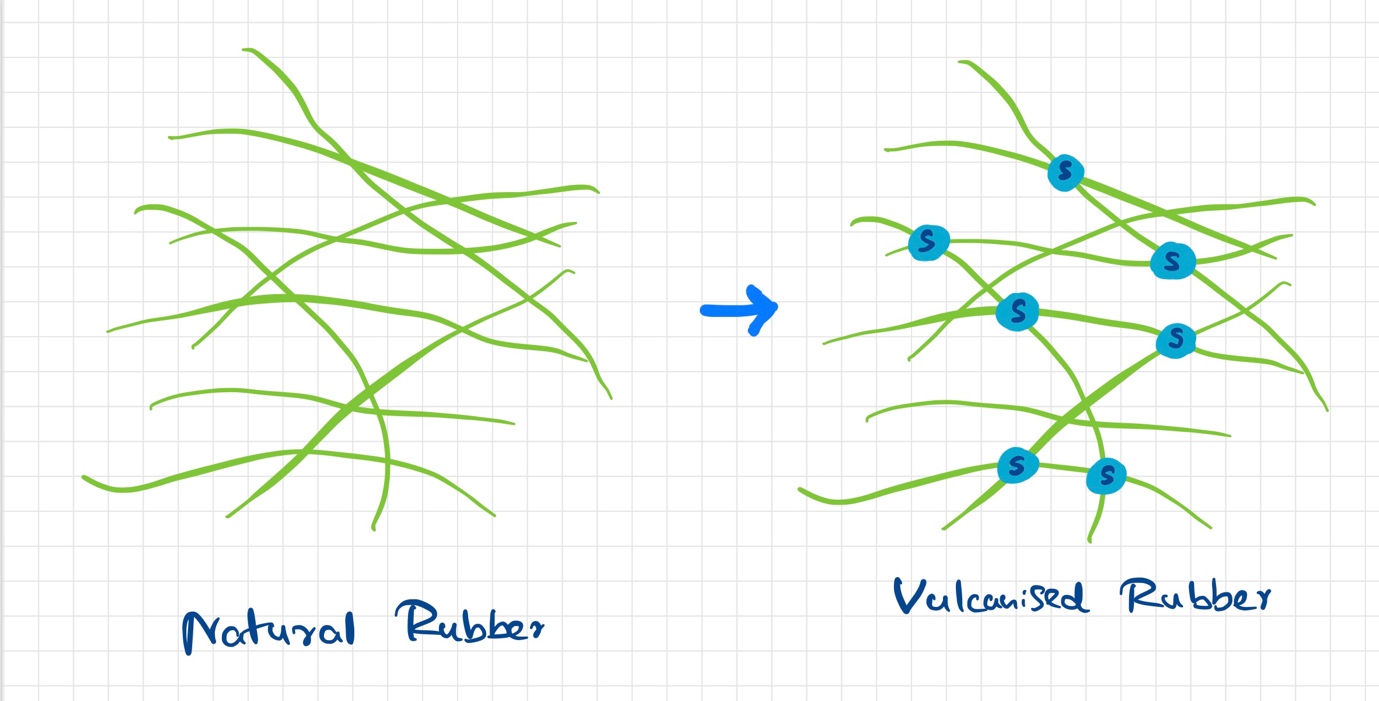
The process of adding sulfur to rubber to improve its properties is called vulcanization. The word has been derived from Vulcan, the Roman god of fire. Vulcanization is a chemical process that includes polymers of rubber being linked to other polymers of rubber. This is done with sulfur bonds acting as bridges between rubber polymers. This process was discovered accidentally in 1839 by Charles Goodyear, who was trying to find a way of preventing the rubber from becoming sticky in hot weather and hard in cold weather.
In the vulcanization of rubber, sulfur is used as the crosslinking agent. The crosslinking reaction takes place between the carbon atoms at the ends of the polymer chains. This reaction is carried out at 140°C or more. At this temperature, free radicals are formed which attack the carbon-carbon double bonds present in the polymer chain and result in crosslinking. The resulting product is referred to as vulcanized rubber.
Why natural rubber needs vulcanization
- Vulcanized rubber is stronger and durable.
- Vulcanized rubber has more tensile strength.
- Vulcanized rubber can withstand higher temperatures.
- Vulcanized rubber is more elastic.
- Plasticity of the vulcanized rubber is relatively low.
- Vulcanized rubber is insoluble in most chemical solvents.
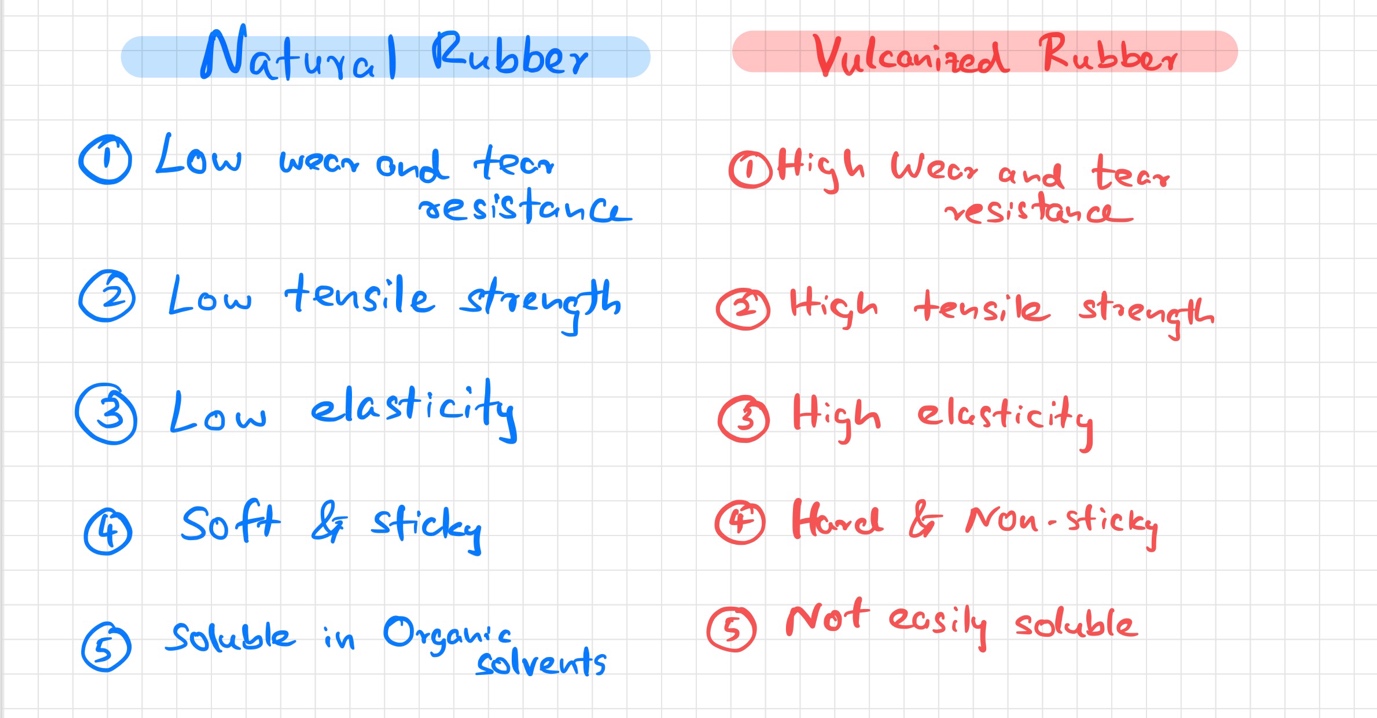
Natural rubber vs Vulcanized rubber
Natural or else crude rubber is soft and sticky with low elasticity and low tensile strength. It has poor resistance to wear and tear and can be damaged by common organic solvents as its soluble in them. The problems are overcome in the process of vulcanization.
Here is a detailed outline of the differences between natural rubber and vulcanized rubber.
How rubber is vulcanized? – The process of vulcanization of rubber
Vulcanization of rubber by sulfur alone is a very slow process. It may take a lot of hours and higher temperatures for this reaction to occur in a significant amount. But maintaining higher temperatures is costly. Higher temperatures can destroy the chemical architecture of the rubber as well. This will result in a poor quality outcome in rubber.
In industrial setting rubber is vulcanized by using accelerators, which are chemical compounds that enhance the reaction efficiency.
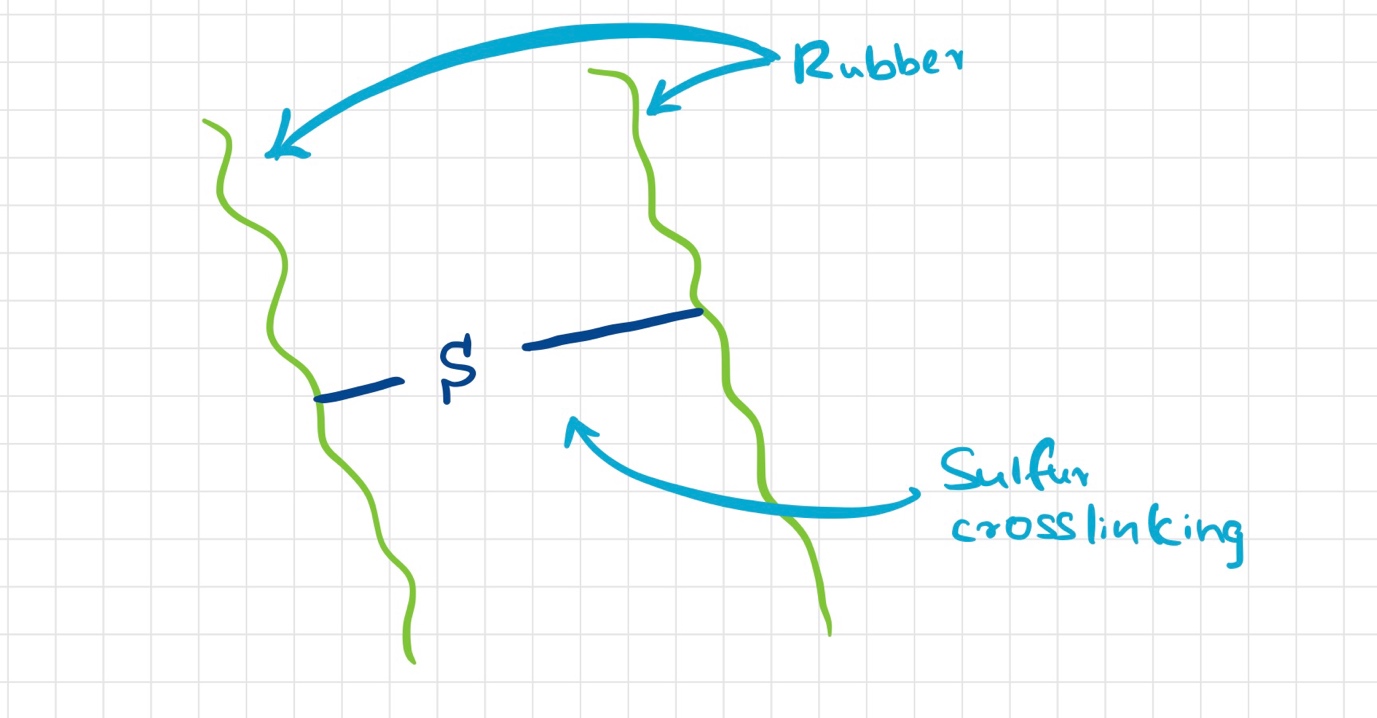
As mentioned above, sulfur-vulcanized rubbers are cross-linked through the formation of carbon-sulfur bonds between polymer chains. The chemical reaction responsible for this cross-linking involves an attack by a thiol group of one polymer chain on a double bond present on another chain.
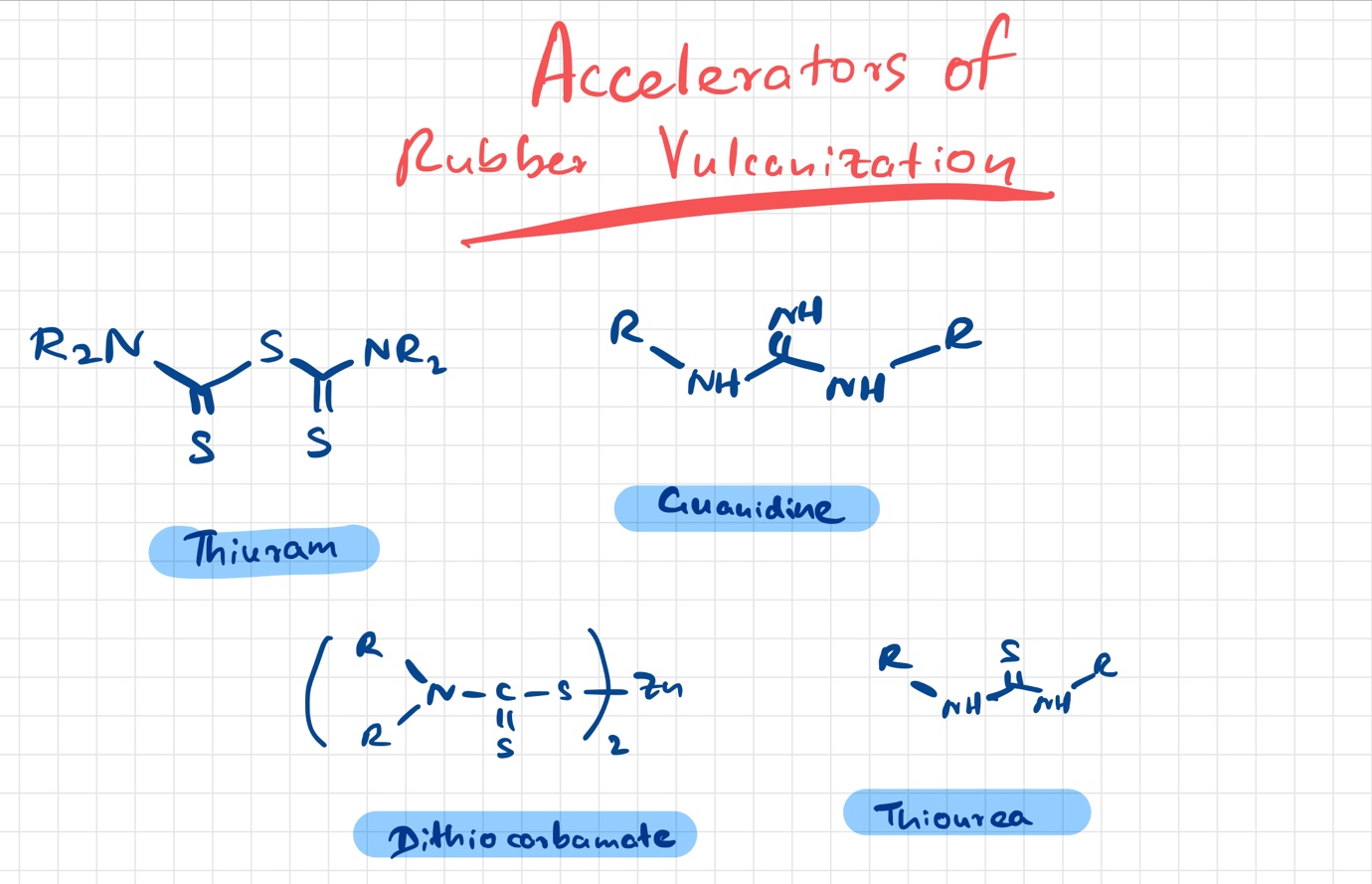
What are accelerators used in vulcanization of rubber
Accelerators are chemical compounds used for speeding up the vulcanization process at relatively lower temperatures. When accelerators are used the crosslinking mechanism is enhanced.
Some of the accelerators can help to crosslink by acting as sulfur donor compounds and it reduces the amount of sulfur that is needed for the vulcanization process.
Primary accelerants speed up the reaction and perform the reaction more efficiently at lower temperatures. Secondary accelerants are needed to enhance the action of primary accelerants. Some accelerants require activators like zinc metal compounds for their optimum function. Accelerants or accelerators are different from catalysts as the latter is not consumed during the chemical reactions.
Rubber and health issues
We have a dedicated article on black rubber mix allergy on our website health article section. Read it here.