Electrolysis is a process in which an electrical current is used to drive a non-spontaneous chemical reaction. It is a powerful tool for transforming chemicals and creating new products.
Electrolytic processes are the chemical transformations caused by passing electricity through a solution or liquid that conducts electricity.
The Electrolysis Process
In electrolysis, a chemical reaction is driven by an external electrical current. The process typically involves two electrodes immersed in an electrolyte solution. The electrodes are connected to a power source, which provides the electrical energy necessary to drive the reaction. The electrodes are made of different materials depending on the reaction being carried out. For example, in the electrolysis of water, the anode is typically made of platinum or other inert material, while the cathode is typically made of a more reactive metal such as copper.
The electrolyte solution is made up of ions that can conduct electricity. When an electrical current is applied to the electrodes, the positive ions are attracted to the negative electrode (cathode), while the negative ions are attracted to the positive electrode (anode). As the ions move towards the electrodes, they gain or lose electrons, depending on their charge. This causes chemical reactions to occur at the electrodes, leading to the formation of new products.
What is an electrolyte?
Electrolytes are liquids or solutions that conduct electricity.
An electrolyte is a substance that conducts electricity when dissolved in a solvent, such as water. Electrolytes are typically in the form of ions, which are atoms or molecules that have gained or lost one or more electrons, giving them a net positive or negative charge. Common electrolytes include sodium, potassium, calcium, and chloride ions.
In a solution containing electrolytes, the ions are free to move around and carry electric charge, allowing the solution to conduct electricity.
Examples for electrolytes
Aqueous solutions of ionic compounds
- Aqueous sodium chloride
- Aqueous copper sulphate
Molten (fused) liquids of ionic compounds
- Fused sodium chloride
Solutions of acids
- Aqueous hydrochloric acid
- Aqueous sulphuric acid
Solutions of bases
- Aqueous sodium hydroxide
Applications of Electrolysis
Electrolysis has numerous applications in industry, research, and everyday life. Here are some commonly seen applications:
Production of Metals:
Electrolysis is commonly used in the production of metals such as aluminum, magnesium, and copper. In these processes, the metal ions are reduced at the cathode, forming a pure metal. For example, in the electrolysis of aluminum, aluminum ions are reduced at the cathode to form aluminum metal, while oxygen gas is evolved at the anode.
Electroplating:
Electroplating is a process in which a thin layer of metal is deposited onto a surface using electrolysis. This process is used to create decorative coatings, prevent corrosion, and improve wear resistance. In the electrolysis of copper sulfate solution, for instance, copper ions are reduced at the cathode to form a thin layer of copper metal on the plating object’s surface.
Chlor-Alkali Industry:
The chlor-alkali industry produces chlorine gas, hydrogen gas, and sodium hydroxide via electrolysis. In this process, a brine solution (sodium chloride) is electrolyzed, producing chlorine gas at the anode, hydrogen gas at the cathode, and sodium hydroxide in the solution.
Electrolytic Cells:
Electrolytic cells are devices that use electrolysis to generate electrical energy. These cells are commonly used in batteries and fuel cells. In a battery, chemical energy is converted to electrical energy through a series of electrochemical reactions. In a fuel cell, electrical energy is generated by the electrochemical reaction of hydrogen and oxygen.
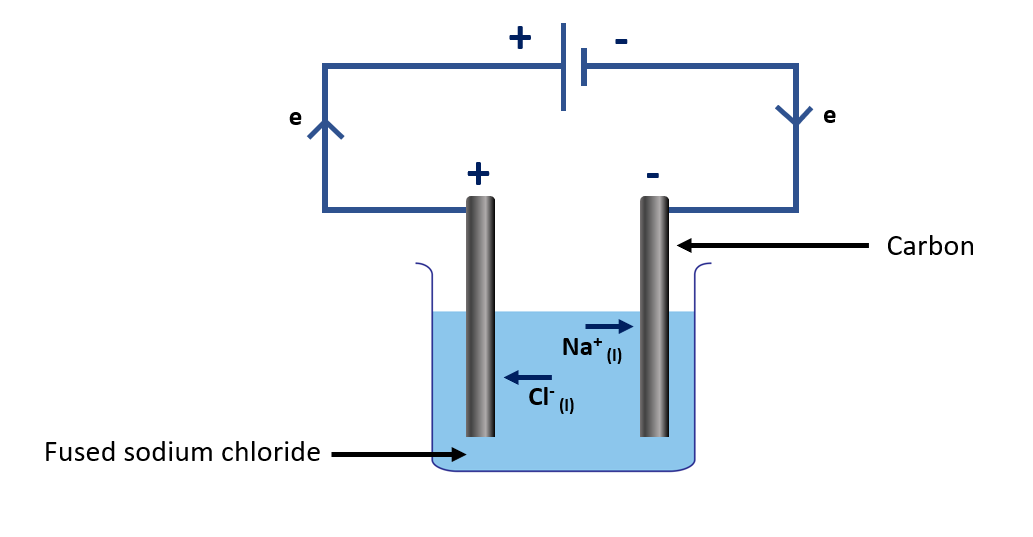
Explain electrolysis of fused sodium chloride using carbon electrodes
Electrolysis of fused sodium chloride using carbon electrodes is a process in which a molten mixture of sodium chloride (NaCl) and calcium chloride (CaCl2) is electrolyzed using carbon electrodes. This process is commonly used in the production of metallic sodium, which has various industrial applications.
During the electrolysis process, the carbon electrodes are immersed in the molten sodium chloride mixture and connected to a power source. The sodium chloride molecules are dissociated into sodium ions (Na+) and chloride ions (Cl–) in the molten mixture. The sodium ions migrate to the negative electrode (cathode), while the chloride ions migrate to the positive electrode (anode).
Electrolysis of fused sodium chloride apparatus
Reaction occurring at the negative electrode (cathode):
At the cathode, the sodium ions gain electrons and are reduced to form sodium metal (Na):
Na+ (molten) + e → Na (molten)
Reaction occurring at the positive electrode (anode):
At the anode, the chloride ions lose electrons and are oxidized to form chlorine gas (Cl2):
2Cl– (molten) → Cl2 (g) + 2e
Overall electrolytic reaction:
The overall electrolytic reaction can be written as:
2NaCl (molten) → 2Na (molten) + Cl2 (g)
This shows that the electrolysis of fused sodium chloride using carbon electrodes produces metallic sodium and chlorine gas as the main products.
The production of metallic sodium through this process is important in many industrial applications, such as the manufacture of sodium compounds, organic synthesis, and in the production of batteries. However, the process requires high temperatures and can be expensive, which limits its widespread use.
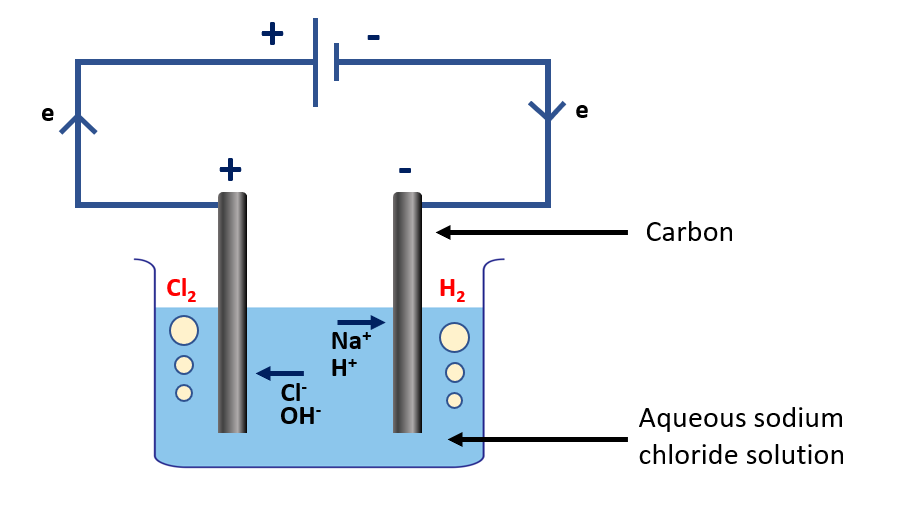
Explain electrolysis of aqueous sodium chloride
Electrolysis of aqueous sodium chloride (NaCl) is the electrolysis of a solution of NaCl in water. This method is frequently used to produce chlorine gas, sodium hydroxide, and hydrogen gas.
During the electrolysis process, two electrodes, typically made of inert materials such as platinum or carbon, are placed in the solution and connected to a power source. The sodium chloride molecules are dissociated into sodium ions (Na+) and chloride ions (Cl–) in the solution. The sodium ions migrate to the negative electrode (cathode), while the chloride ions migrate to the positive electrode (anode).
Electrolysis of aqueous sodium chloride apparatus
Reaction occurring at the negative electrode (cathode):
Na+ and H+ ions in the solution move towards the negative electrode. As hydrogen is below sodium in the activity series, it is the H+ ions that are reduced here.
2H+(aq) + 2e 🡪 H2(g)
Reaction occurring at the positive electrode (anode):
At the anode, the chloride ions lose electrons and are oxidized to form chlorine gas (Cl2)
2Cl–(aq) → Cl2 (g) + 2e
overall electrolytic reaction
2 H+(aq) + 2Cl– (aq) 🡪 H2(g) + Cl2(g)
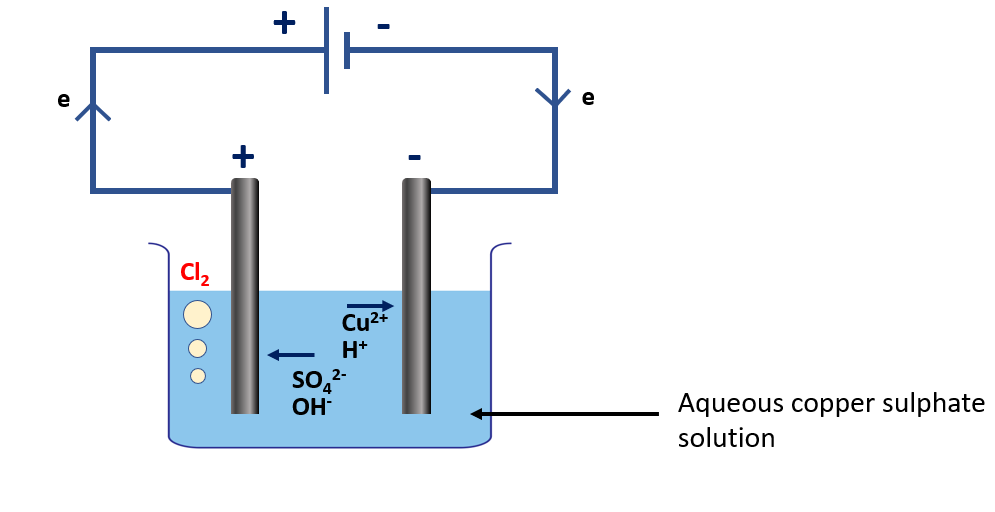
Explain electrolysis of an aqueous copper sulphate solution
Electrolysis of an aqueous copper sulfate (CuSO4) solution is a process in which a solution of CuSO4 in water is electrolyzed. This process is commonly used in the purification and electroplating of copper.
During the electrolysis process, two electrodes, typically made of inert materials such as platinum or graphite, are placed in the solution and connected to a power source. The CuSO4 molecules are dissociated into copper ions (Cu2+) and sulfate ions (SO42-) in the solution. The copper ions migrate to the negative electrode (cathode), while the sulfate ions migrate to the positive electrode (anode).
Electrolysis of an aqueous copper sulphate solution apparatus
Reaction occurring at the negative electrode (cathode):
At the cathode, the copper ions gain electrons and are reduced to form solid copper metal (Cu):
Cu2+ (aq) + 2e → Cu (s)
Reaction occurring at the positive electrode (anode):
At the anode, the sulfate ions lose electrons and are oxidized to form oxygen gas (O2) and water (H2O):
4OH– (aq) → O2 (g) + 2H2O (l) + 4e
Overall electrolytic reaction:
The overall electrolytic reaction can be written as:
CuSO4 (aq) → Cu (s) + SO42- (aq)
This shows that the electrolysis of aqueous copper sulfate produces solid copper metal and sulfate ions as the main products.
Numerous industrial applications, such as the production of electrical wires, plumbing fixtures, and other copper-based products, rely on the products of this process. This process can also be used to purify copper of impurities and electroplate copper onto other surfaces.
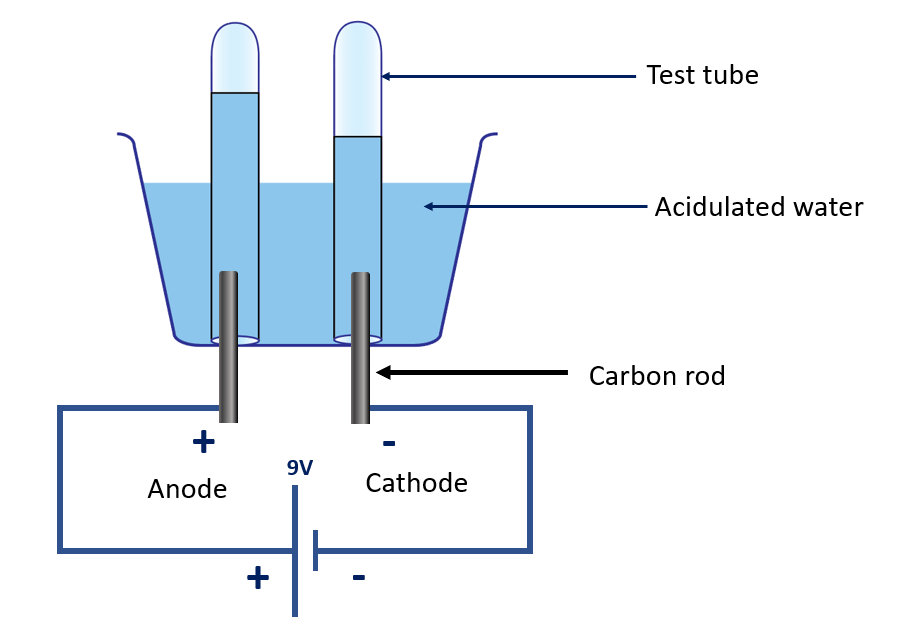
Explain electrolysis of acidulated water
Electrolysis of acidulated water is a process in which water (H2O) containing a small amount of acid, typically sulfuric acid (H2SO4), is electrolyzed. This process is used in many industrial applications, such as the production of hydrogen gas, oxygen gas, and the purification of metals.
During the electrolysis process, two electrodes composed of inert substances such as platinum or graphite are placed in acidulated water and connected to a power source. The water molecules are dissociated into hydrogen ions (H+) and hydroxide ions (OH–) in the solution. The hydrogen ions migrate to the negative electrode (cathode), while the hydroxide ions migrate to the positive electrode (anode).
Electrolysis of acidulated water apparatus
Reaction occurring at the negative electrode (cathode):
At the cathode, the hydrogen ions gain electrons and are reduced to form hydrogen gas (H2):
2H+ (aq) + 2e → H2 (g)
Reaction occurring at the positive electrode (anode):
At the anode, the hydroxide ions lose electrons and are oxidized to form oxygen gas (O2) and water (H2O):
4OH– (aq) → O2 (g) + 2H2O (l) + 4e
Overall electrolytic reaction:
The overall electrolytic reaction can be written as:
2H2O (l) → 2H2 (g) + O2 (g)
This demonstrates that the main products of electrolysis of acidulated water are hydrogen gas and oxygen gas.
The products of this process are important in many industrial applications, such as the production of fuels, chemicals, and electricity. Additionally, this process can be used to purify metals, such as copper and aluminum, by using the hydrogen gas produced to reduce the metal ions and form pure metal at the cathode.