Manganese is an element that has the chemical symbol Mn and the atomic number 25. It belongs to the group of transition metals and is located in the periodic table between chromium and iron. It was first isolated as a pure metal by the Swedish chemist Johan Gottlieb Gahn in 1774.
Manganese is a hard, silvery-gray metal that is essential to many biological and industrial processes. It is a key component of many alloys, including steel, and is used in the production of batteries, ceramics, and pigments. Manganese is also used as a catalyst in a variety of chemical reactions.
Manganese has a wide range of oxidation states, which allows it to participate in many different chemical reactions. It forms a variety of compounds with oxygen, sulfur, and other elements. One of the most important of these compounds is manganese dioxide (MnO2), which is widely used as a catalyst and in the production of dry cell batteries.
Manganese is an important nutrient for humans and other animals, playing a crucial role in many biological processes, including bone formation, metabolism, and wound healing. However, excessive exposure to manganese can be toxic, particularly to the nervous system. Chronic exposure to high levels of manganese can cause a neurological disorder known as manganism, which shares many similarities with Parkinson’s disease.
In the end, manganese is a useful and important element that has a wide range of uses in both industry and biology. Its unique properties make it a useful part of many different materials and processes, but to use it safely, it needs to be handled carefully.
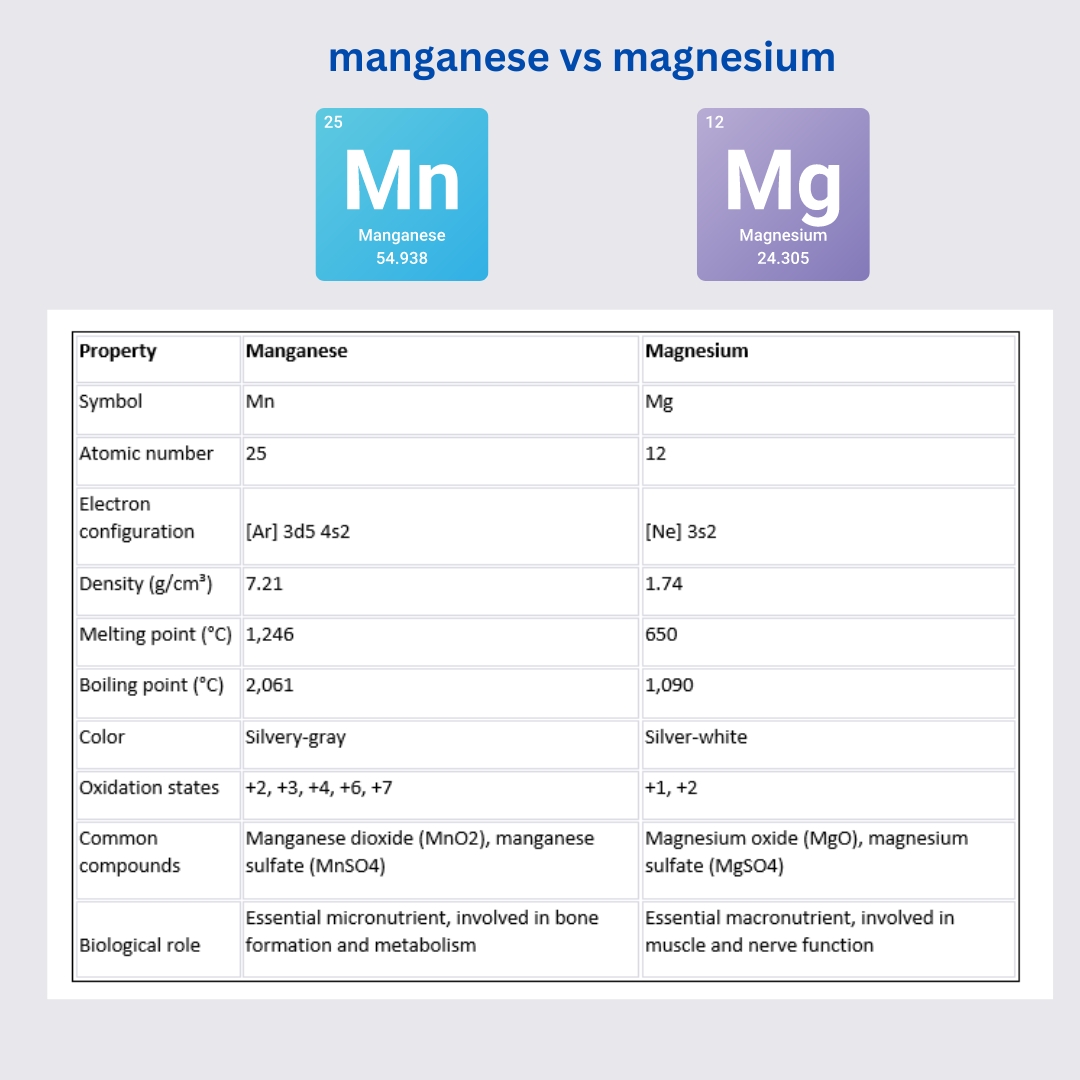
manganese vs magnesium
| Property | Manganese | Magnesium |
|---|---|---|
| Symbol | Mn | Mg |
| Atomic number | 25 | 12 |
| Electron configuration | [Ar] 3d5 4s2 | [Ne] 3s2 |
| Density (g/cm³) | 7.21 | 1.74 |
| Melting point (°C) | 1,246 | 650 |
| Boiling point (°C) | 2,061 | 1,090 |
| Color | Silvery-gray | Silver-white |
| Oxidation states | +2, +3, +4, +6, +7 | +1, +2 |
| Common compounds | Manganese dioxide (MnO2), manganese sulfate (MnSO4) | Magnesium oxide (MgO), magnesium sulfate (MgSO4) |
| Biological role | Essential micronutrient, involved in bone formation and metabolism | Essential macronutrient, involved in muscle and nerve function |
common reactions of manganese and magnesium
Manganese and magnesium are both metallic elements that have different chemical and physical properties, resulting in different reactions.
Common reactions of Manganese:
- Manganese reacts with oxygen in the air to form manganese dioxide (MnO2).
Reaction with oxygen in the air: 2 Mn + O2 → 2 MnO2
- Manganese reacts with hydrochloric acid to form manganese(II) chloride (MnCl2) and hydrogen gas (H2).
Reaction with hydrochloric acid: Mn + 2 HCl → MnCl2 + H2
- Manganese reacts with sulfuric acid to form manganese(II) sulfate (MnSO4) and hydrogen gas (H2).
Reaction with sulfuric acid: Mn + H2SO4 → MnSO4 + H2
- Manganese reacts with sodium hydroxide to form manganese(II) hydroxide (Mn(OH)2).
Reaction with sodium hydroxide: Mn + 2 NaOH → Mn(OH)2 + H2
- Manganese reacts with water to form manganese(II) oxide (MnO) and hydrogen gas (H2).
Reaction with water: Mn + 2 H2O → MnO + H2
Common reactions of Magnesium:
- Magnesium reacts with oxygen in the air to form magnesium oxide (MgO).
Reaction with oxygen in the air: 2 Mg + O2 → 2 MgO
- Magnesium interacts with hydrochloric acid to generate magnesium chloride (MgCl2) and hydrogen gas (H2).
Reaction with hydrochloric acid: Mg + 2 HCl → MgCl2 + H2
- Magnesium acts with sulfuric acid to make magnesium sulfate (MgSO4) and hydrogen gas (H2).
Reaction with sulfuric acid: Mg + H2SO4 → MgSO4 + H2
- Magnesium reacts with water to form magnesium hydroxide (Mg(OH)2) and hydrogen gas (H2).
Reaction with water: Mg + 2 H2O → Mg(OH)2 + H2
Difference:
- Manganese reacts with water to form manganese(II) oxide and hydrogen gas, while magnesium reacts with water to form magnesium hydroxide and hydrogen gas.
Mn + 2 H2O → MnO + H2
Mg + 2 H2O → Mg(OH)2 + H2
- Manganese is a transition metal, while magnesium is an alkaline earth metal.
- Manganese has a higher melting point and boiling point than magnesium.
- Manganese has a greater tendency to form complexes due to its partially filled d-orbitals, while magnesium does not readily form complexes.
- Manganese is more reactive than magnesium due to its higher electronegativity and ability to form more stable compounds.
Different oxides of manganese, symbol and uses
| Oxide of Manganese | Symbol | Uses |
|---|---|---|
| Manganese(II) oxide | MnO | Used as a catalyst, in the production of steel and ceramics, and in batteries. |
| Manganese(III) oxide | Mn2O3 | Used as a pigment in paints and ceramics, and in the production of other manganese compounds. |
| Manganese(IV) oxide | MnO2 | Used as a catalyst, in the production of dry cell batteries and electronic components, and in the production of glass and ceramics. |
| Manganese(V) oxide | Mn2O5 | Used as a catalyst and in the production of other manganese compounds. |
What is special about manganese nodules?
Manganese nodules, also known as polymetallic nodules, are small, potato-shaped rocks that are found on the ocean floor. These nodules are rich in manganese, but also contain other metals such as iron, nickel, copper, and cobalt, among others.
What is special about these nodules is that they contain high concentrations of valuable metals, making them a potential source of these metals for various industries. However, they are difficult and expensive to extract, and there are environmental concerns associated with their extraction.
Manganese nodules are also important to the ecosystem of the world’s oceans because they provide homes for many different kinds of animals. They can also take in carbon and other things, which helps keep the ocean’s chemistry in balance.
manganese steel
Manganese steel, also known as Hadfield steel, is a type of alloy steel that is composed of a combination of manganese and iron. It is known for its high impact strength, toughness, and ability to work-harden under stress. This makes it ideal for use in applications where durability and resistance to wear and tear are important, such as in the construction of mining and quarrying equipment, rock crushers, and railway switches.
The unique properties of manganese steel are due to the high percentage of manganese in its composition, which can range from 11% to 14%. This high level of manganese content allows for the formation of a surface-hardening effect, which occurs when the steel is subjected to impact or pressure. As a result, the steel becomes harder and more resistant to wear and tear over time, making it an excellent choice for heavy-duty applications.
Manganese steel is strong and durable, but it can’t be used in high-temperature situations because it can become brittle and lose its toughness when it gets too hot. Because it is hard and gets harder with use, it is also harder to machine and weld than other types of steel.