Generation of electricity
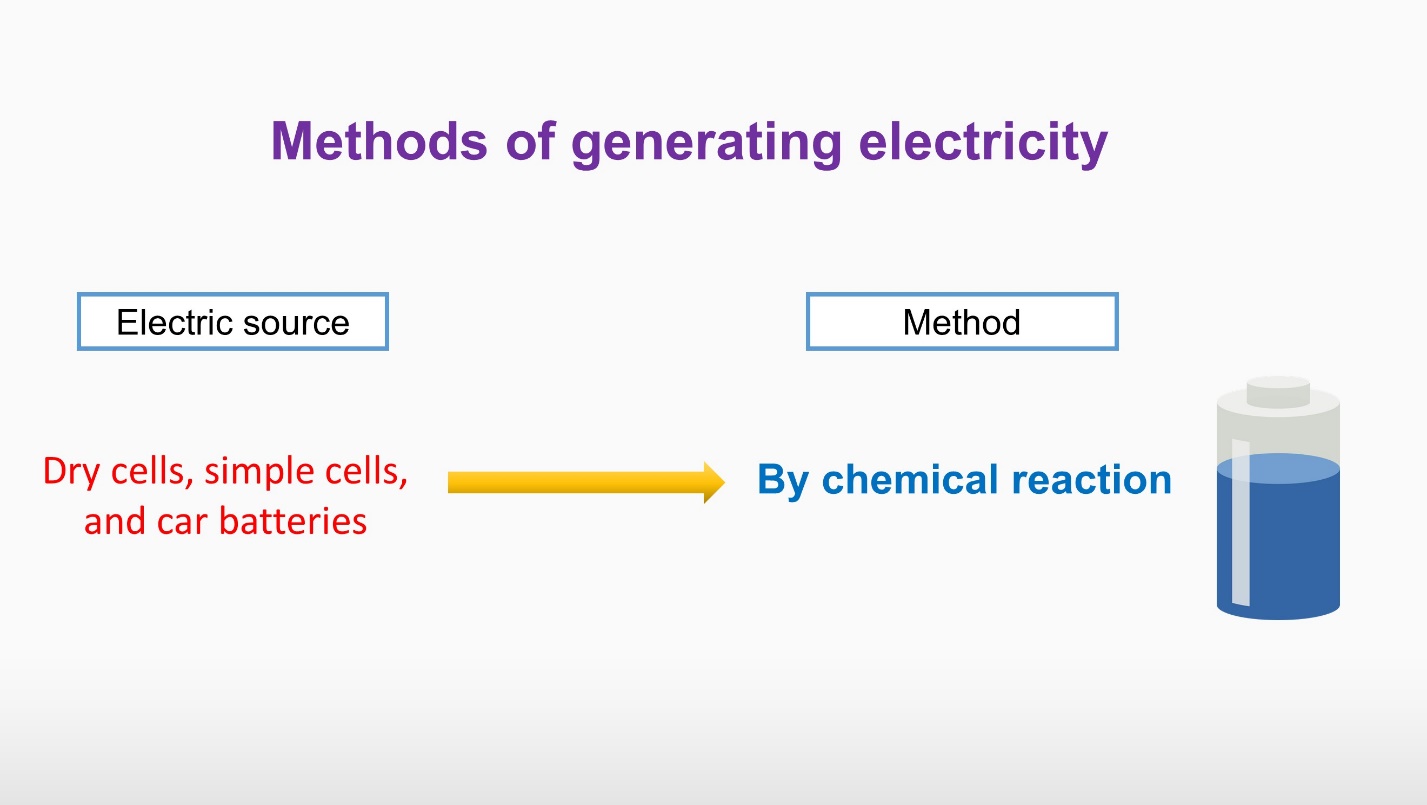
Some sources can generate electricity by chemical reactions. Examples are Dry cells, simple cells, and car batteries.
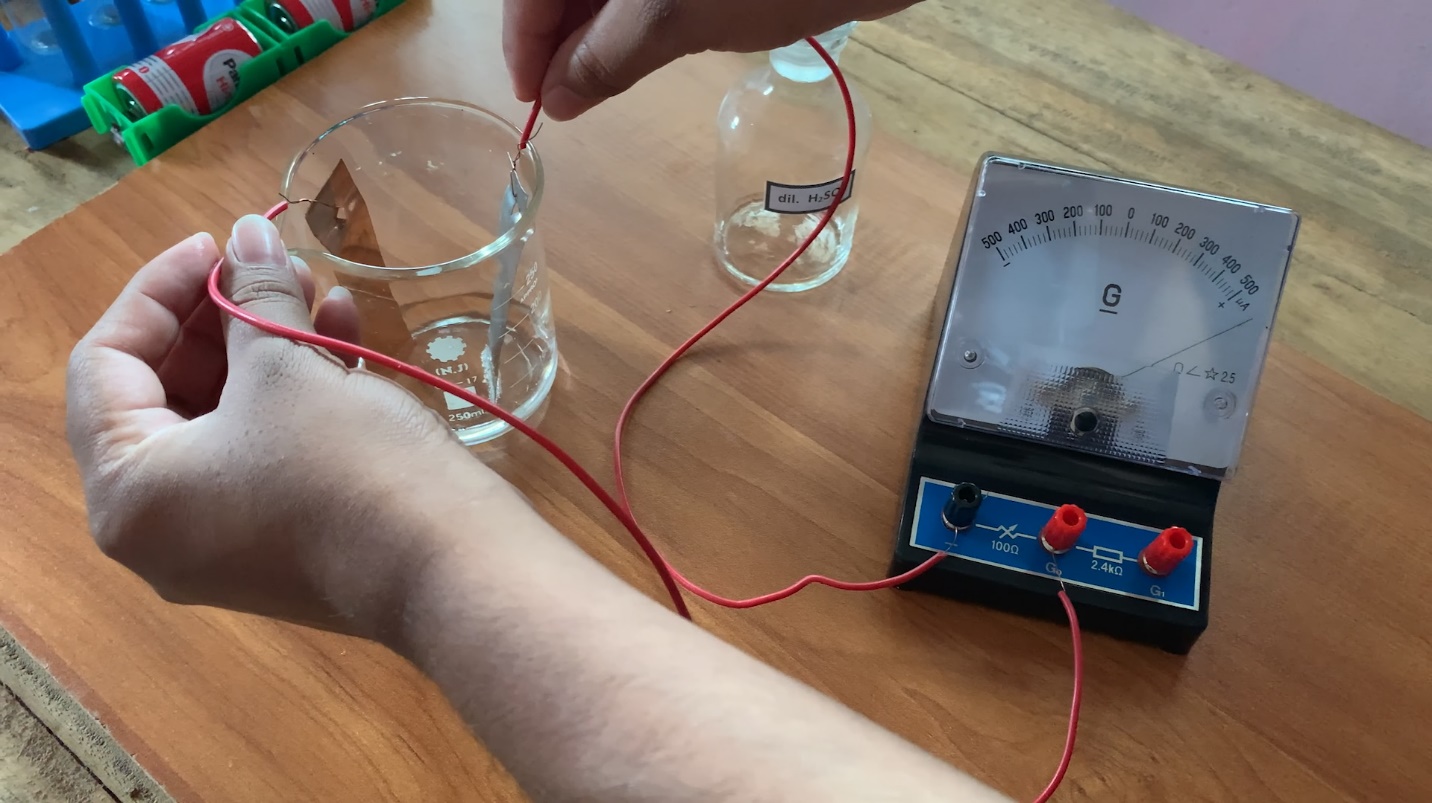
Let’s make a simple cell.
You will need
A small beaker, copper, and zinc sheets, a torch bulb, a center-zero galvanometer, dilute sulphuric acid, and pieces of connection wire.
Method
- Thoroughly clean the copper and zinc sheets and attach a piece of wire to one end of each sheet.
- Half-fill the beaker with dilute acid.
- Dip copper and zinc sheets into the acid without allowing them to come into contact.
- The positive and negative terminals of the galvanometer should be connected to the copper and zinc sheets respectively.
- The copper sheet is considered positive and the zinc sheet is considered the negative terminal of the simple cell.
Observations
- The indicator of the galvanometer deflected
- The evolution of gas bubbles neared the zinc sheet.
Conclusion
- The generated electric current is shown by the indicator of the galvanometer.
Weaknesses of a simple cell
Simple cells are not in daily use due to the disadvantages in them. Some of them are,
- The difficulty of using them – because it contains liquids.
- Inability to obtain current for a longer period.
Nowadays, there are cells and batteries that can produce more current and last longer. They have replaced the simple cell in daily use.