Introduction
To learn the basics of human biology, you need to have a good understanding of the functions of carbon dioxide. It is one of the most important gases that are produced by our body and it plays a vital role in maintaining homeostasis.
The carbon dioxide levels in our body are increased when we breathe out air without inhaling fresh oxygen. When this happens, the cells produce more CO2 than usual and remove excess amounts from the blood through bicarbonate ion transport mechanism. In this blog post, we will discuss how bicarbonate transports excess levels of carbon dioxide from one place to another.
Carbon Dioxide Transport in human body
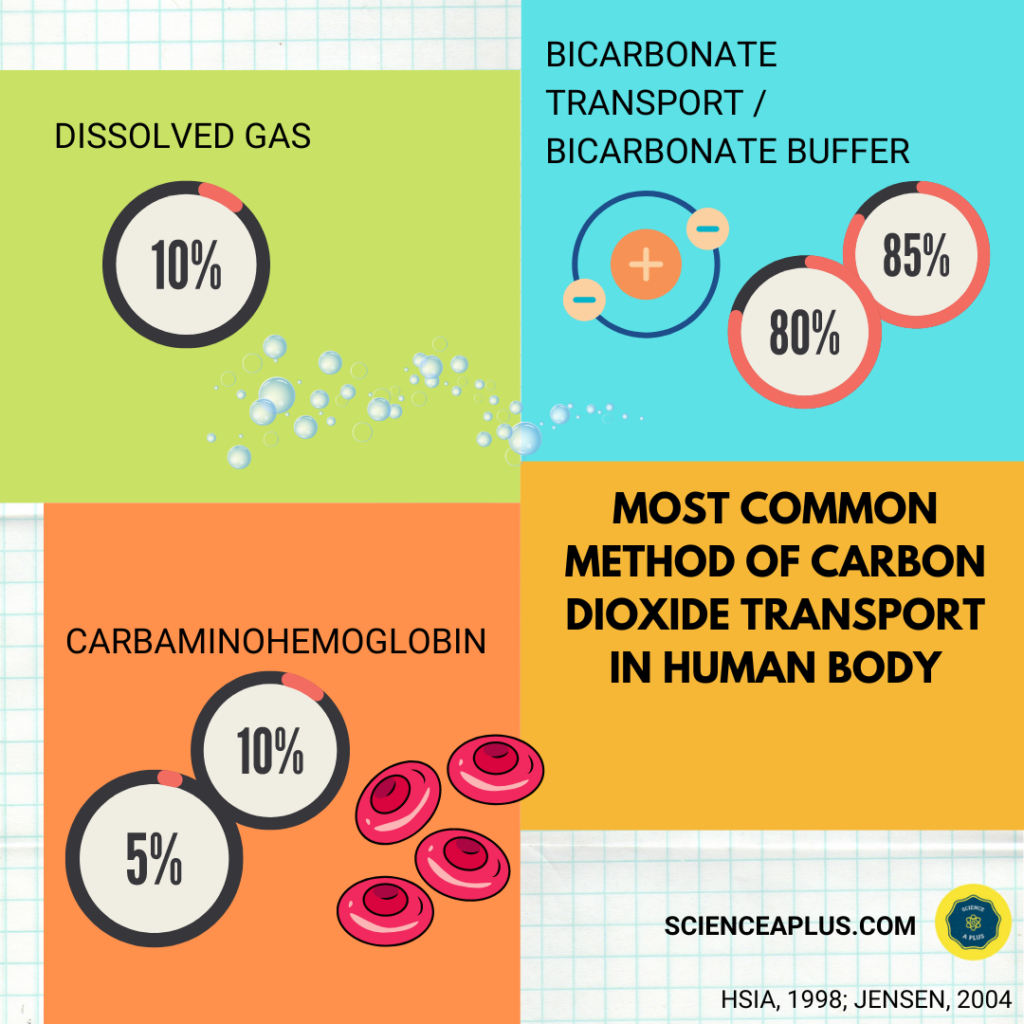
Carbon dioxide is transported in the bloodstream from peripheral tissues and back to the lungs in three ways.
First, carbon dioxide is dissolved in blood plasma. This is called “dissolved gas.”
Second, when bicarbonate ions are added to the circulatory system, they are converted into carbon dioxide and water by the enzyme carbonic anhydrase. This process is called “bicarbonate transport / bicarbonate buffer”
Third, when hemoglobin (along with other proteins) forms carbaminohemoglobin. This happens when carbon dioxide binds with haemoglobin molecules.
Carbon Dioxide Transport in blood as bicarbonates
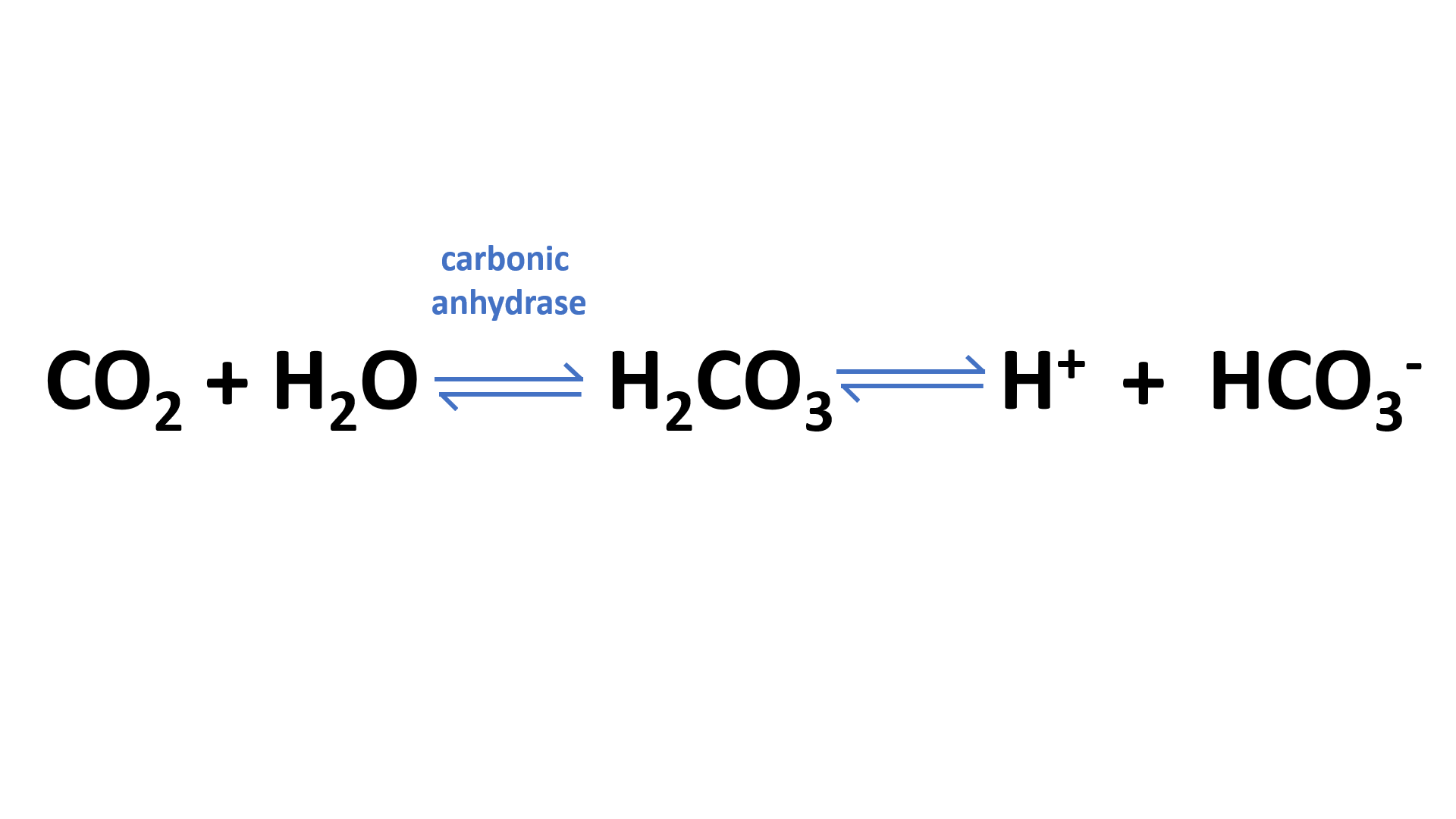
Carbon dioxide (CO2) is transported in the blood through a process called the carbonic anhydrase reaction, which describes how CO2 dissociates into carbonic acid (H2CO3).
The hydrogen ions from this reaction are then carried back to the lungs by one of two methods: they can be carried on hemoglobin as bicarbonate ions or dissolved in plasma and carried as hydrogen ions. Bicarbonate ions released in the lungs combine with water to form carbonic acid again, which then becomes CO2 once more.
Carbon dioxide excretion
Carbon dioxide is a byproduct of cellular respiration. Cellular respiration is the main mechanism that produces ATP energy to all the living cells in the human body.
Carbon dioxide is mainly excreted in two pathways.
- Exhaling air removes carbon dioxide from body as CO2
- Kidneys excrete bicarbonate ions (HCO3)– to maintain acid base balance in human body
When the carbon dioxide levels increase in our body
Carbon dioxide levels rise in respiratory acidosis, a condition in which respiratory power is not enough to excrete carbon dioxide from body.
When the carbon dioxide levels increase in our body, it dissociates into hydrogen (H+) and bicarbonate (HCO3)– by reacting with water inside cells. The increased hydrogen ion is carried back to the lungs.
The (HCO3)– is transported to the kidneys. In the kidneys excess bicarbonate ions are excreted into urine.
The bicarbonate ion (HCO3)– can be found in many different parts of the body such as blood plasma and intracellular fluid. It also plays an important role when it comes to maintaining certain pH levels within our system as well as keeping our bones healthy by helping them absorb calcium from food we eat.
Bicarbonate ion plays an important role in transporting CO2 from one place to another
Bicarbonate ion plays an important role in transporting CO2 from one place to another. It is produced by the lungs and released into the blood as bicarbonate ion. In addition, it is also released from red blood cells into tissues where it can be used to alkalize acidic extracellular fluid. This process continues until a normal pH level is reached.
Conclusion
In this way, you can understand that bicarbonate ion is the most common method of carbon dioxide transport in human body.
So, this was all about the way carbon dioxide is transported in our body. Now you know how bicarbonate ion plays an important role in transporting CO2 from one place to another.