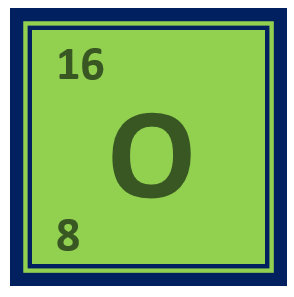
Oxygen is a chemical element – Its symbol is O and the atomic is number 8. It is a non-metal and is the third most abundant element in the universe, after hydrogen and helium. Below is an overview of the chemical and physical properties of oxygen, its valency, electronic configuration, uses, reactions with other elements, and industrial and medical applications.
Chemical Properties of Oxygen:
- Oxygen is highly reactive and forms oxides with most elements, including metals and non-metals.
- It is a vital component of many organic and inorganic compounds.
- Oxygen is involved in many combustion reactions, such as the burning of fuels.
Physical Properties of Oxygen:
- Oxygen is a colorless, odorless gas at room temperature and pressure.
- It has a boiling point of -183 °C and a melting point of -218.4 °C.
- Oxygen is slightly soluble in water and other polar solvents.
- It is a non-metal and is not ductile or malleable.
Valency of Oxygen:
Oxygen has a valency of 2, meaning it can form two bonds with other elements.
It typically forms covalent bonds with other non-metals and ionic bonds with metals.
Electronic Configuration of Oxygen:
Oxygen has an electron configuration of [He] 2s2 2p4.
It has six valence electrons and is two electrons short of a full octet.
Uses of Oxygen:
Oxygen gas is used in many industrial processes, such as steel production and waste treatment.
It is used in medicine to treat breathing problems and as a supplement for patients with low oxygen levels.
Oxygen is a critical component in many chemical reactions, including combustion and oxidation.
Reactions of Oxygen with Other Elements:
Oxygen readily reacts with many elements, particularly metals, to form oxides.
It is involved in many combustion reactions, such as the burning of fuels.
Oxygen can also form compounds with nitrogen to form nitrogen oxides, which are pollutants.
Balanced equations for Reactions of Oxygen with Other Elements
Oxygen + Hydrogen -> Water
2 H2 + O2 -> 2 H2O
Oxygen + Carbon -> Carbon Dioxide
C + O2 -> CO2
Oxygen + Nitrogen -> Nitrogen Oxides
N2 + O2 -> 2 NO or 2 NO2 (depending on conditions)
Oxygen + Sulfur -> Sulfur Dioxide
S + O2 -> SO2
Oxygen + Iron -> Iron Oxide
4 Fe + 3 O2 -> 2 Fe2O3
Oxygen + Magnesium -> Magnesium Oxide
2 Mg + O2 -> 2 MgO
Industrial Uses of Oxygen:
Oxygen is used in many industrial processes, such as steel production, chemical synthesis, and waste treatment.
It is used in welding and cutting, as well as in some types of propulsion systems.
As an oxidizer, oxygen is a crucial component of rocket fuel.
Medical Uses of Oxygen:
Oxygen is used in medicine to treat breathing problems and to supplement patients with low oxygen levels.
It is used in some medical equipment, such as oxygen concentrators and ventilators.
Oxygen is used in hyperbaric oxygen therapy, which involves breathing pure oxygen in a pressurized chamber to increase oxygen levels in the body.
Chemical and Physical Properties of Oxygen in a table
| Property | Description |
| Chemical Symbol | O |
| Atomic Number | 8 |
| Valency | 2 |
| Electron Configuration | [He] 2s2 2p4 |
| Physical State | Gas |
| Boiling Point | -183 °C |
| Melting Point | -218.4 °C |
| Density | 1.429 g/L at STP |
| Color | Colorless |
| Reactivity | Highly reactive |
| Solubility | Slightly soluble in water and polar solvents |