Preparing a Biuret solution
Biuret reagent is a commonly used solution for detecting the presence of proteins in a sample. The biuret reagent can be made by adding NaOH to a solution of CuSO4 to make the solution alkaline.
- Dissolve 1.5 g of copper sulfate pentahydrate (CuSO4.5H2O) in 100 mL of distilled water.
- In a separate container, dissolve 6 g of sodium potassium tartrate (Rochelle salt) in 100 mL of 2 M sodium hydroxide (NaOH) solution.
- Mix the two solutions together and dilute with distilled water to a final volume of 1 liter.
- The resulting solution is the Biuret reagent.
The Biuret reagent should be freshly prepared before use, and should be stored in a tightly sealed bottle to prevent contamination. The solution should be discarded if it turns blue or green, as this indicates the presence of copper oxide, which can interfere with the protein test.
Starch test
Materials needed:
A beaker, watch glass, tripod, Bunsen burner, some leaves that are exposed to the sun light well, alcohol, boiling tube, iodine solution, forceps
Method :
- Boil the leaves after dipping them in hot water.
- Then place them in a boiling tube with alcohol and boil in a water bath until the leaves are colorless.
- Remove the leaves and thoroughly wash them. Place them on the watch glass and apply iodine drops to the leaves.
- Take note of the observations.
When iodine is applied to the leaves, they turn a dark blue color. Iodine is an indication that becomes dark blue when starch is present. As a result, this experiment shows that when the necessary conditions are met, the leaves perform photosynthesis and create starch.
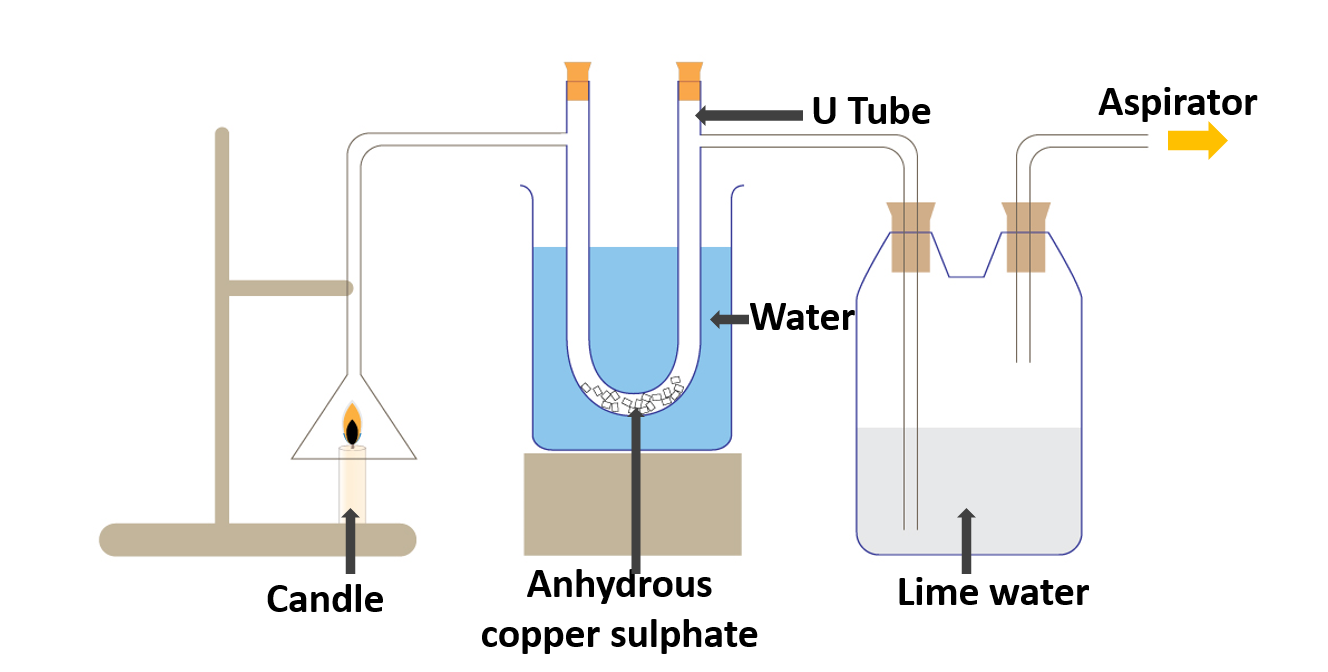
Identify the products formed during the combustion of fuels
A candle, a boiling tube, a bottle, a funnel, lime water, copper sulphate
Method:
- Assemble the apparatus as illustrated in the diagram.
- Connect the aspirator to the boiling tube/bottle with lime water.
- Light the candle and Turn on the aspirator.
- When the aspirator is turned on, an air stream is drawn from the funnel to the boiling tube.
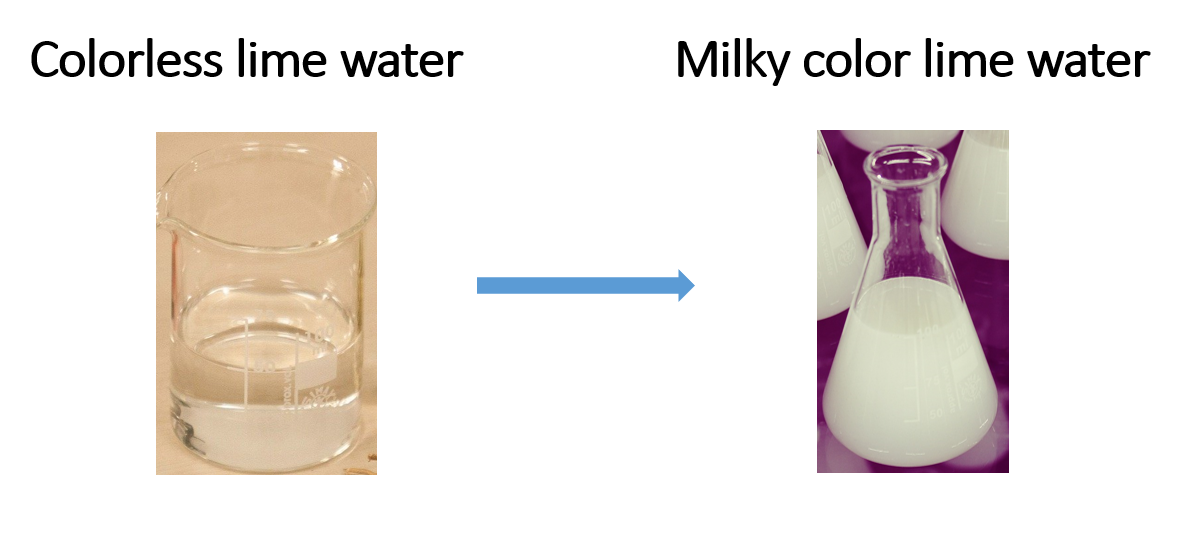
Anhydrous copper sulphate is present in the U tube (white). The boiling tube/bottle is filled with colorless lime water. When the candle is lit and the aspirator is turned on, the white anhydrous copper sulphate turns blue. It may also be noticed that the lime water turns milky.
Read more:
The water (water vapour) drawn into the U tube causes the white anhydrous copper sulphate to turn blue. Carbon dioxide gas causes lime water to turn milky.
This activity shows that when a candle burns, it emits water and carbon dioxide gas. Water and carbon dioxide gas are thus created as byproducts of fuel combustion.
Investigate the factors essential for rusting of iron
Investigate air essential for rusting of iron
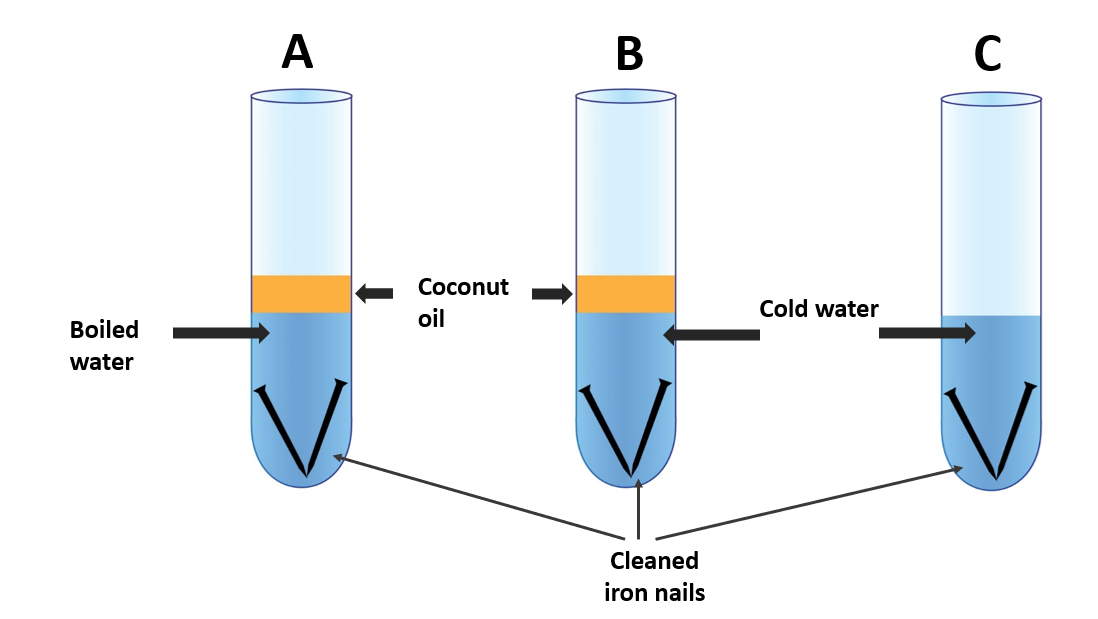
Materials needed:
Three test tubes, iron nails that have been cleansed, and coconut oil
Method:
- Fill a test tube halfway with water and bring it to a boil.
- Insert a clean iron nail into it and coat the water’s surface with oil (setup A).
- When water cools, an oil layer is applied to prevent air from dissolving.
- Fill two other test tubes with equal amounts of cold water and insert a clean iron nail into each. Apply a layer of oil to one of them (setup B).
- Leave the other test tube alone (set-up C).
- After a few days, have a look at the setups.
- Make a note of your findings.
Test tube A’s nail does not rust. All of the air dissolved in it has been removed because it includes boiled water. Adding a coating of coconut oil to water has stopped air from dissolving as the water cools.
Cold water is in test tube B. As a result, its water contains air. Because water contains dissolved air, the nail rusts.
The nail in test tube C is exposed to the outside world. Rusting occurs as it obtains air from the outside. As a result, it can be argued that air is required for rusting.
Investigate moisture essential for rusting of iron
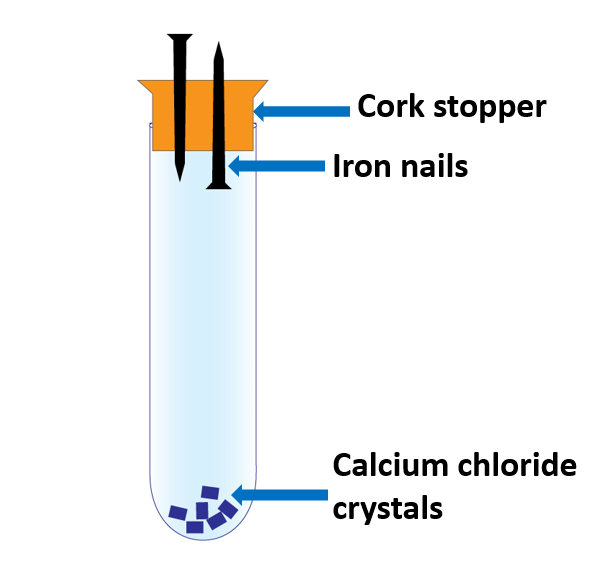
A boiling tube, two cleaned iron nails, cork stopper, calcium chloride crystals, wax, coconut oil
Method:
- Sandpaper should be used to clean the two iron nails.
- Attach them to the cork stopper as illustrated in Figure.
- Fill the boiling tube with calcium chloride crystals and secure the stopper with iron nails.
- Wax the tube to make it airtight.
- Keep an eye on this setup for a few days.
- Make a note of your findings.
After a few days, the parts of the nails outside the boiling tube have rusted whereas the ones inside the tube have not corroded.
In the boiling tube, calcium chloride crystals absorb moisture from the air. Placing wax around the stopper seals the tube and prevents moisture from entering the tube. The parts of nails inside the tube do not rust because the air inside the tube is free of moisture.
Read more:
The goal of driving the two nails into the cork in different directions is to ensure that neither the sharp tip nor the flat head of the nails rust.
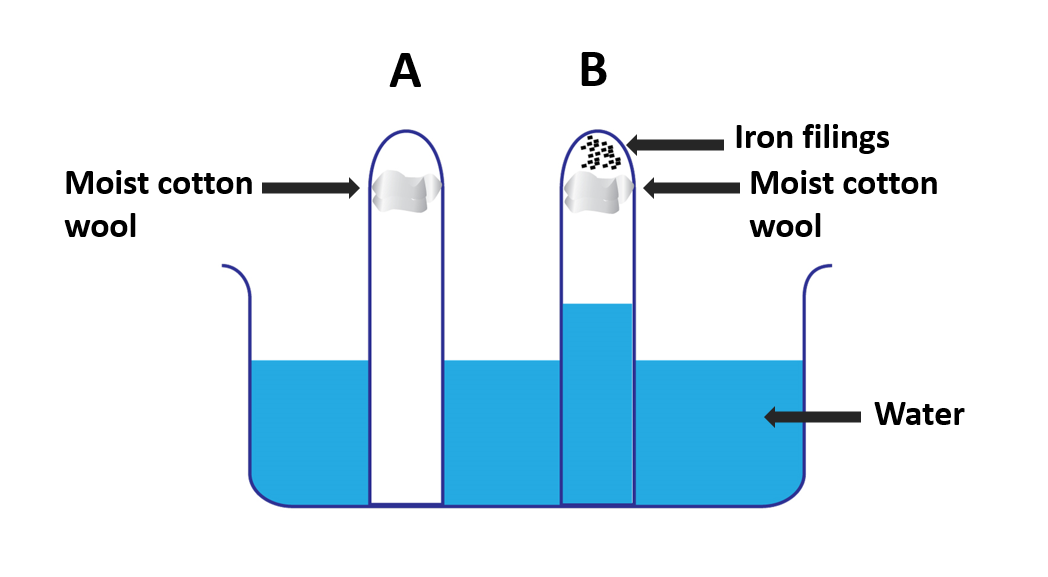
Investigate oxygen in air essential for rusting of iron
Materials needed:
A beaker, two test tubes, iron filings, cotton wool
Method:
- Consider two test tubes. Trap some moist cotton wool in one of them (A). Trap a similar lump of moist cotton wool with some iron filings in the opposite tube (B).
- Fill a beaker halfway with water and immerse the two inverted test tubes A and B in it.
- Keep an eye on this setup for a few days.
- Make a note of your findings.
It can be seen that the iron filings in tube B have rusted while the water has risen to about one-fifth of its original height.
Read more :
The oxygen content of air is 21% by volume. That is, oxygen makes up about one-fifth of the air in a given place. If oxygen gas is used up for rusting, one-fifth of the volume of air in the place where rusting happens should have been consumed.
The oxygen gas in the air in tube B is used up during the rusting of iron filings. Because oxygen makes up one-fifth of the volume of air, the water level rises to one-fifth the height of the test tube. It is evident from this that oxygen gas is consumed during rusting.
These experiments demonstrate that oxygen and water vapour/water in the air are required for iron rusting.
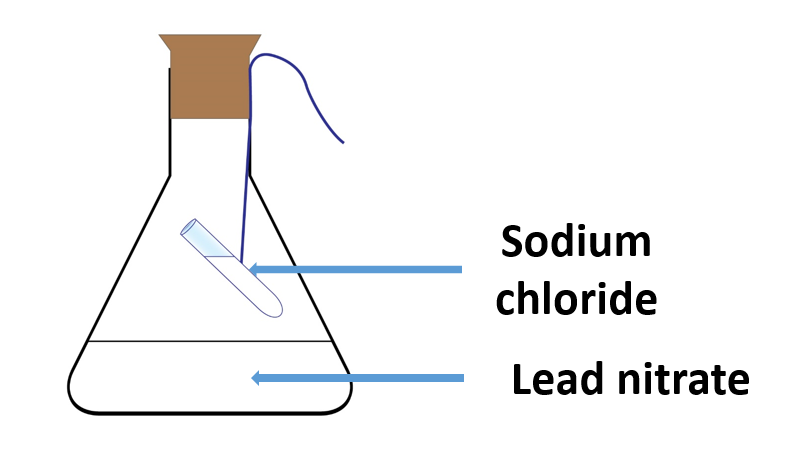
Demonstrate the law of conservation of mass
Materials needed:
A conical flask, lead nitrate 1 g, water 20 ml, sodium chloride 1 g, a boiling tube
Method:
Fill a conical flask halfway with water and dissolve 1g of lead nitrate.
Fill a test tube with 1g of sodium chloride, dissolve it in 5ml of water, and transfer the solution to an ignition tube.
Sodium chloride should be used to tie the ignition tubing.
Using a stopper, hang the solution with a string inside the conical flask containing the lead nitrate solution, as shown in Figure.
The formation of a white precipitate when the two solutions are mixed suggests the presence of a chemical reaction in the instrument.
The experiments also reveal that there is no difference in total mass before and after the reaction.
Read more:
The French scientist Antoine Lavoisier (1743 -1794) demonstrated for the first time in connection to diverse chemical reactions that the entire mass of the substances participating in the reaction (reactants) is equal to the total mass of the products formed after the reaction. This discovery became known as the Law of Conservation of Mass.
Law of conservation of mass
The overall mass of a chemical process remains constant. This signifies that the mass has been conserved.
Heating lead nitrate – Formation of a red powder
(Evolution of a brown-coloured gas)
Materials needed:
A boiling tube, a bunsen burner, and lead nitrate
Method:
- Add approximately 1g of lead nitrate to a boiling tube.
- The Bunsen burner is used to heat the boiling tube.
- Make a note of your findings.
When white lead nitrate is heated, a brown gas is produced, leaving a crimson powder in the boiling tube. This is a chemical alteration because the composition of lead nitrate has changed.
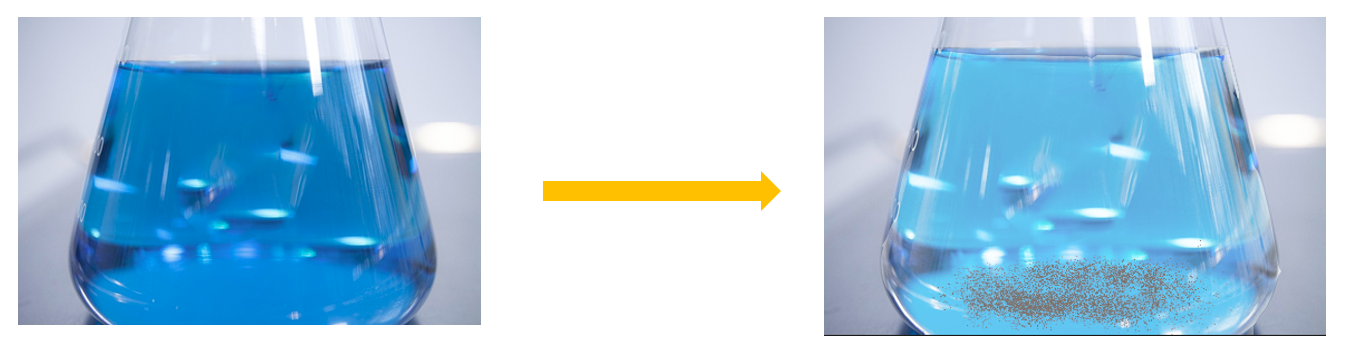
Putting an iron nail into a copper sulphate solution
Materials needed:
A thermometer, copper sulphate, an iron nail, a boiling tube
Method:
- Prepare a light blue solution by adding water and copper sulphate crystals to the boiling tube.
- Insert the cleaned iron nail.
- Make a note of your findings.
When a cleaned iron nail is immersed in a copper sulphate solution, the blue color of the solution fades and a reddish brown substance forms on the nail and at the bottom as the temperature rises.
Study microbial activities
Materials needed:
Sugar, yeast, a balloon, warm water (40 oC), a bottle (500 ml), a beaker/suitable container
Method:
- In 200 mL of warm water, dissolve two teaspoons of sugar.
- Pour one teaspoon of yeast into the sugar solution described above.
- Leave it for about 20 minutes and then check it.
- Pour a freshly prepared solution into the bottle next.
- Then attach a balloon to the bottle’s mouth.
- After around 20 minutes, make a note of your observations.
When sugar and yeast are combined in a solution, it begins to bubble and warm up. You will detect the aroma of alcohol. It is because ethyl alcohol is created as a result of yeast activity in sugar solution.
The balloon has been inflated, as you can see. This is due to the gas released by yeast action in sugar solution. Carbon dioxide is the gas produced here.
Read more:
Yeast is an important element in baked goods. Yeast action produces carbon dioxide, which causes the dough to rise. Ethyl alcohol evaporates throughout the baking process.
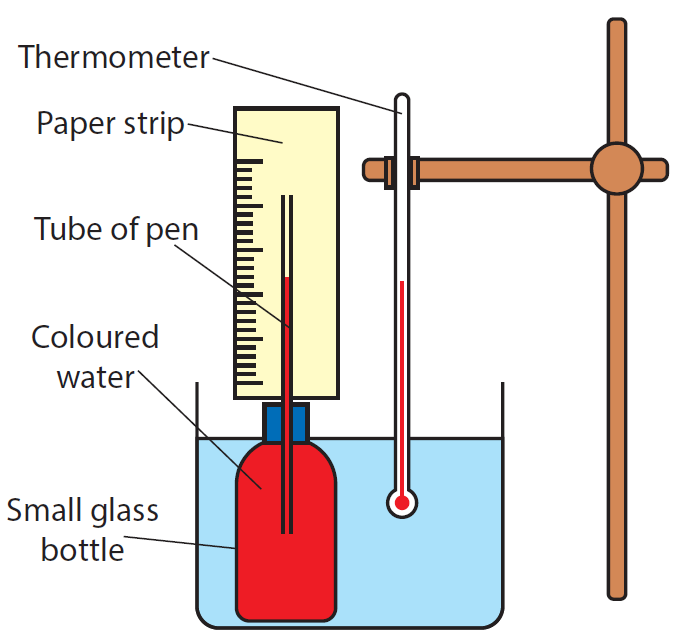
How to make a simple thermometer
Materials needed:
A small glass container is filled with colored water and a ball point pen’s empty tube is inserted. A paper strip on which a scale is marked, is glued to the tube.
Method:
- A little glass container is filled with colored water and a ball point pen’s empty tube is inserted.
- A paper strip with a scale on it is pasted to the tube.
- The filled bottle is placed in a beaker of water and slowly heated.
- A mercury thermometer is immersed in a water beaker.
The temperature of the water rises as it is heated, and the coloured water column in the pen tube rises as well.
For some temperature readings reported by the thermometer, the upper end of the coloured water column is marked on the paper strip. At the mark, the relevant temperature should also be specified. A basic scale can be built by marking multiple temperatures on the paper strip. The resulting water-bottle-thermometer can be used to measure unknown temperatures over a short distance. You may receive a rough estimate here.
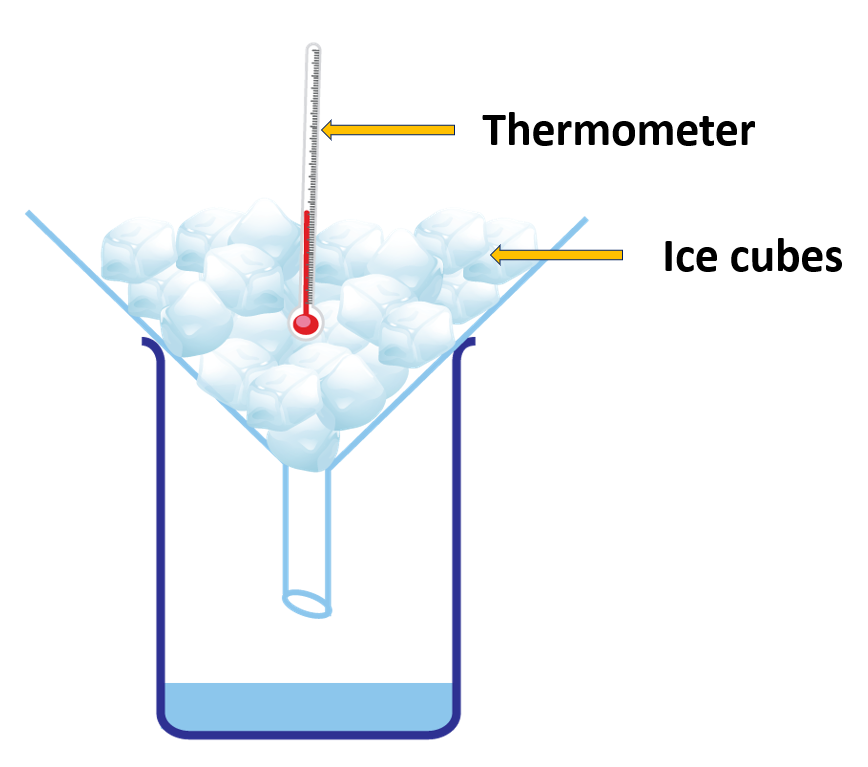
Determine the constant temperature at which ice turns to water.
(Find out the melting point of water)
Materials needed:
A boiling tube, a beaker, some ice chips, water, a thermometer, a burner, a stand, a stirer
Method:
- Approximately one quarter of a boiling tube should be occupied by ice chips.
- Put the apperatus in its proper place.
- Apply enough heat to for the ice to melt.
- Utilizing a stirrer, thoroughly mix the water together.
- Tabulate temperature against time.
You’d see that the temperature remains constant until all of the ice melts.
Read more:
When heat is applied, matter changes from solid to liquid without increasing its temperature. The melting point is the specific temperature at which this change in state happens.
In the previous experiment, the temperature remained constant at 0 oC until all of the ice melted. So, under normal atmospheric pressure, the melting point of pure water is 0 oC.
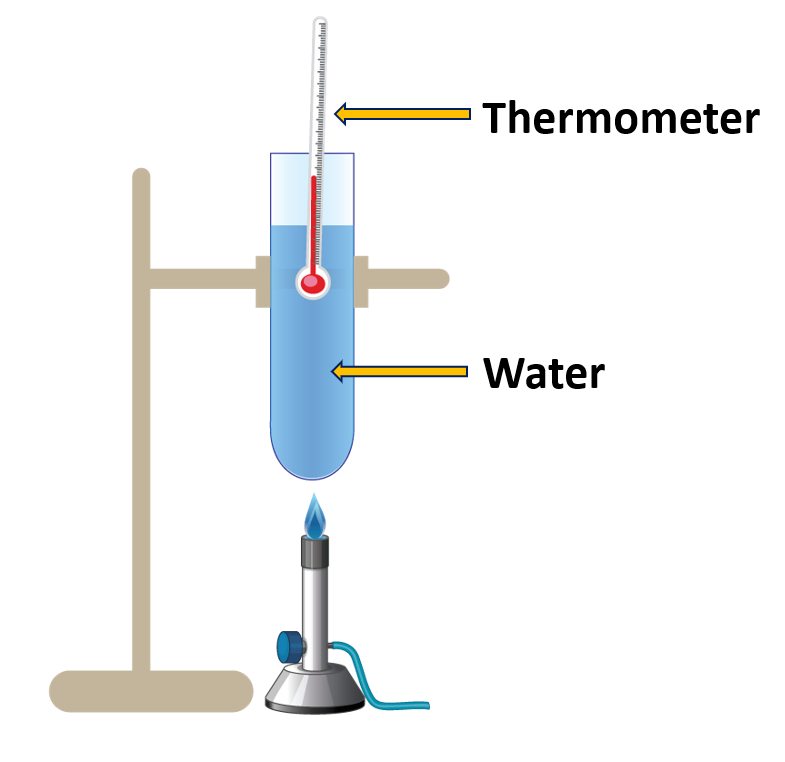
Find out the boiling point of water
Materials needed:
A boiling tube, water, a thermometer, a stand, a burner
Method:
- Fill a boiling tube with water and install a thermometer as illustrated in Figure.
- The burner is used to heat the water.
- Measure the temperature change over time.
When water is heated, the temperature gradually rises. At some point, the temperature rise stops and water transitions from the liquid to the vapour state. That temperature remains constant until all of the water has evaporated. This temperature is referred to as the boiling point of water.
Read more:
At standard atmospheric pressure, the boiling point of pure water is 100 oC.The boiling point of a liquid is affected by its surroundings.. The boiling point decreases as the surrounding pressure decreases. (On a high mountain, the boiling point of water is less than 100 degrees Celsius).
If water is not pure due to foreign substance dissolution, the boiling point (100 oC) may be raised or lowered. As a result, it is evident that the boiling point is a physical property that may be used to determine the purity of a substance.
Phenomenon that happens due to the radiation
Materials needed:
Three equal tin cans, three thermometers, cork stoppers, black and white paint, cold water, a few pieces of cardboard, and a stop clock are all included.
Method :
- Keep one tin can with the shining outside surface.
- Paint one of the remaining tin cans black and the final one white.
- Fill each tin with an equal amount of cold water.
- Attach a thermometer to each tin and record the starting temperature.
- Put all three cans in the same spot in the sun.
- Measure the temperature every five minutes and keep track of the results.
After some time, the temperature of the water in the black can has increased above that of the other two cans. Furthermore, the temperature of the water in the can with the gleaming outer surface has risen extremely slowly.
Solar radiation heats the water in the tin cans. It is obvious that dark absorbs radiated heat quickly, whereas polished bright surfaces absorb it slowly. In addition, white surfaces absorb less radiated heat than black surfaces.
How to identify water in laboratory?
- Copper sulphate, a blue chemical, is found in the laboratory.
- It becomes white when heated. Anhydrous copper sulphate is white-colored copper sulphate.
- When water is introduced to anhydrous copper sulphate, the color returns to blue.
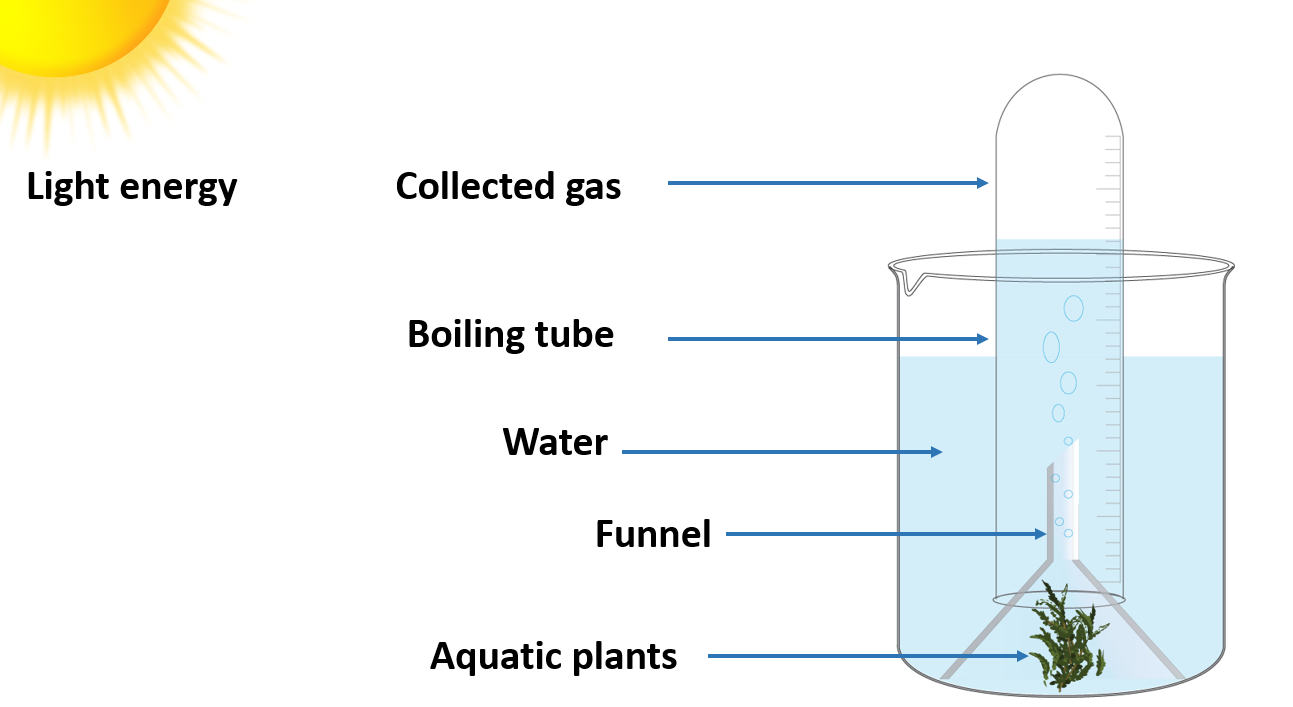
Study that oxygen is produced during photosynthesis
A funnel, some aquatic plants, water, a trough, a boiling tube, and a glowing splinter
Method :
- Fill the jar halfway with water and keep several Hydrilla plants in it. Insert the funnel.
- Fill the boiling tube with water and close the free end of the funnel.
- Allow the setup to be exposed to sunshine.
- Take note of your observations.
- Carefully remove the boiling tube and insert a glowing splinter into it.
- Keep an eye on what happens.
You will notice that air bubbles released by the plants collect in the upper portion of the test tube. You must carefully remove the test tube and insert the glowing splinter. The glowing splinter will relight and burst into flame, confirming the presence of oxygen inside the test tube. As a result, you can conclude that oxygen is produced during photosynthesis.
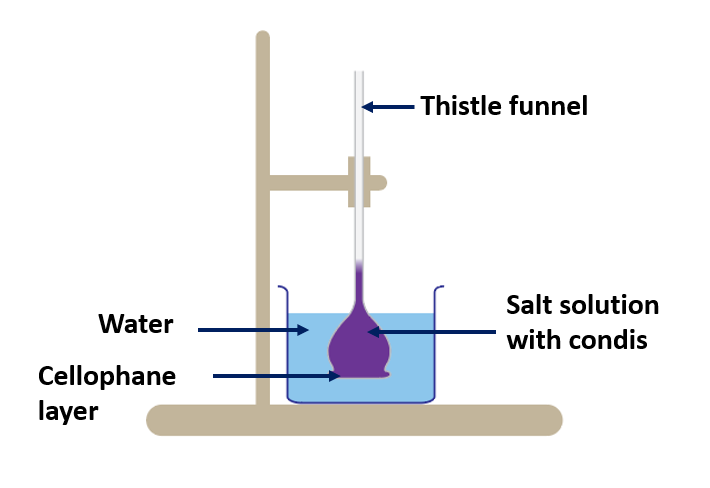
The mechanism through which water particles move from cell to cell.
(Demonstration of osmosis)
Materials needed:
A thistle funnel, a piece of colorless cellophane, a 500 ml beaker, rubber band threads, salt solution, water, and condis solution are all required.
Method:
- Fill the beaker partially with water.
- Cover the funnel’s mouth with a piece of cellophane.
- Place the funnel in the beaker and fill it with condis solution and salt solution.
- In the thistle funnel, mark the starting level.
- After a few minutes, take note of your observations.
After a few minutes, you will notice that the liquid level in the funnel rises and the color of the condis fades.
The reason for the increase in liquid level is discussed below.
The concentration of water molecules in the beaker is higher than in the solution in the thistle funnel. As a result, water molecules prefer to flow across the cellophane from a higher concentration area (beaker) to a lower concentration area (thistle funnel). Cellophane lets just water molecules to pass through while keeping salt and condis molecules out. Semipermeable membranes are the name given to such membranes.
Read more:
Only certain molecules can flow through a semipermeable barrier. You can replicate the experiment with an egg membrane instead of cellophane. Egg membrane is a semipermeable membrane as well.
What is osmosis?
Diffusion of water molecules through a semipermeable membrane from an area of higher water concentration to an area of lower water concentration is known as osmosis.
What happens when you mix copper sulfate solution with sodium hydroxide solution?
Materials needed:
A solution of copper sulphate, a solution of sodium hydroxide, two test tubes
Method:
- Mix the copper sulphate and sodium hydroxide solutions.
- Make a note of your findings.
When the copper sulphate solution is mixed with the sodium hydroxide solution, a light blue solid is formed. Precipitates are such solids.
What happens when a granule of zinc is added to hydrochloric acid?
Materials needed:
Dilute hydrochloric acid, a zinc granule, a boiling tube
Method:
- Fill the boiling tube partially with dilute hydrochloric acid.
- Put the zinc piece into it.
- Make a note of your findings.
When a granule of zinc is put in hydrochloric acid, it dissolves and a gas is released.