What is boron nitride?
Boron nitride is a white, odorless powder. It is used as an additive to make the steel harder and less brittle. Boron nitride can be found in brake pads of cars, airplanes, and in oil well drilling equipment. Along with these uses, boron nitride also has medical use in orthopedic surgery to help the healing of bones.
Boron Nitride is a hardening material with good flowability and fine particle size distribution, making it excellent in the applications of plastic molding and heat transfer material. Besides plastic, boron nitride has also been used in coating applications such as paints, ceramics, adhesives, laminating, etc. Boron nitride also appears to be an effective thermal barrier and wear-resistant material, making it an excellent choice for products utilizing these properties, such as plastics, electrical insulators, lubricants, etc.
What would boron nitride structure look like?
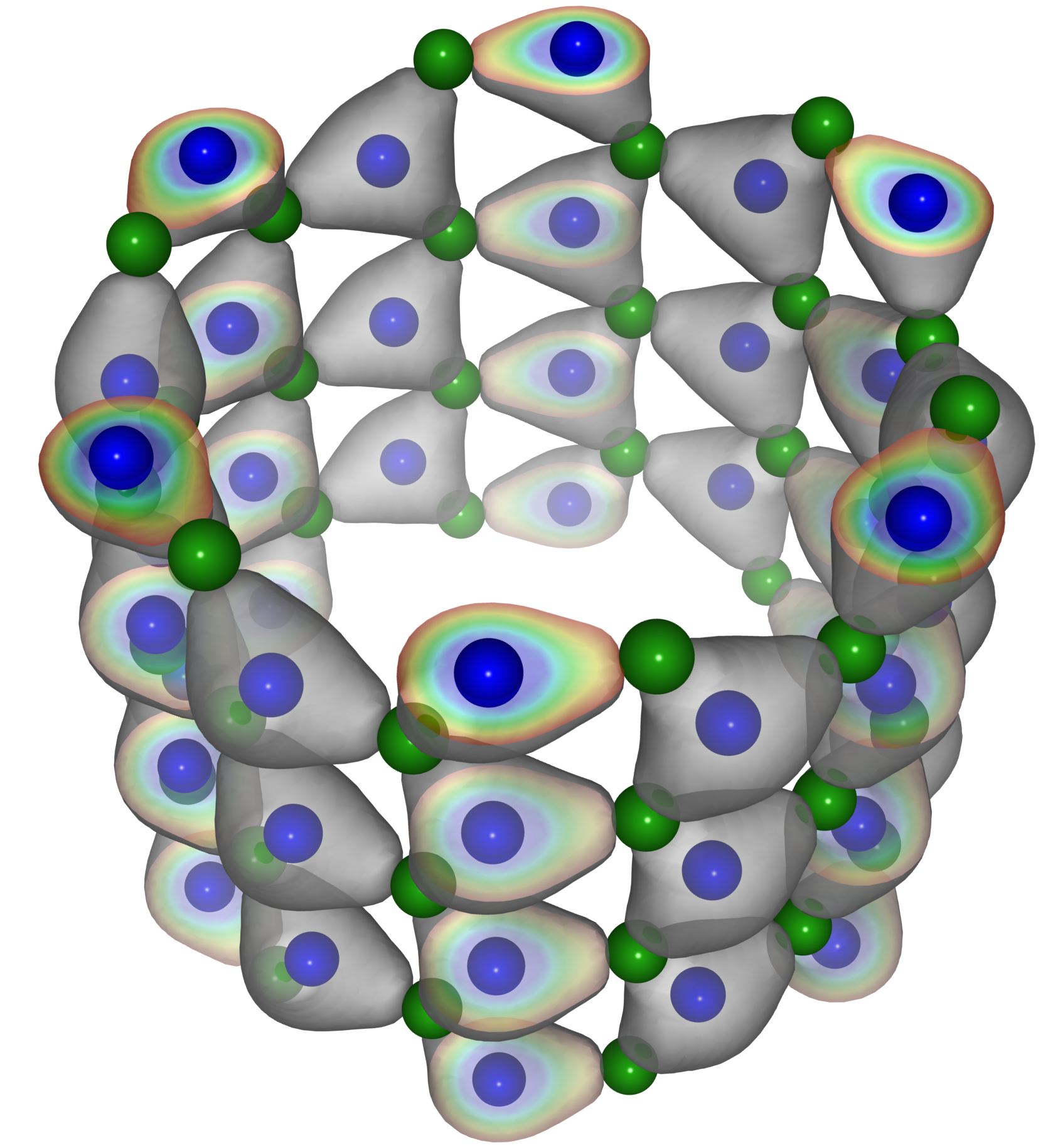
Boron nitride molecule formula is BN. Its chemical structure is a trigonal pyramid. The most common form of Boron nitride is the hexagonal crystal structure. This type of Boron Nitride consists of 600 amu formula units.
The most common forms of Boron Nitride are the cubic and hexagonal types. The most common crystal structure of Boron nitride is the hexagonal type which consists of 600 amu formula units. Each atom of Boron has a partial charge of -1,2 ions resulting in an overall charge of the molecule -2. When boron nitride is heated above 600°C, it forms a transparent plasma which provides good thermal transfer due to its transparency.
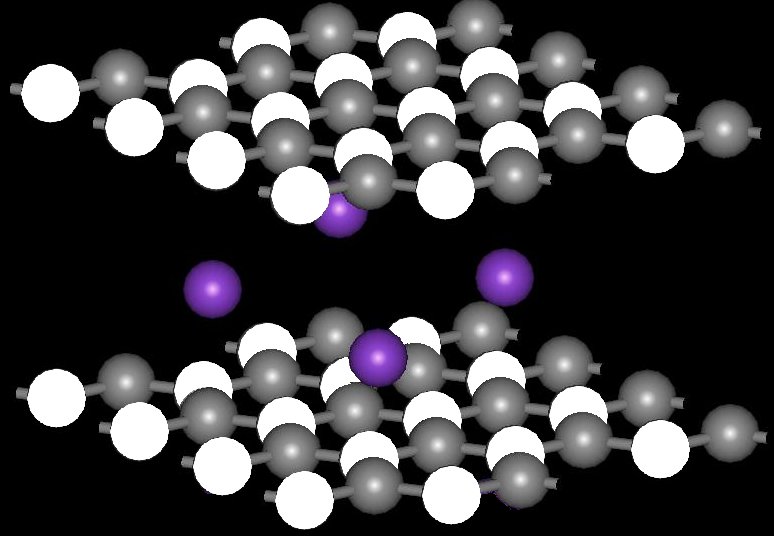
Boron Nitride can be synthesized by a liquid phase reaction between Boron and Nitrogen. This reaction is carried out by heating the reaction mixture in a sealed capsule in an electric furnace at 1700°C for 16 hours. Boron nitride consists of sheets of boron atoms with nitrogen atoms in between the layers. Each layer is separated by less than 3 nanometers taking up only about 10% of the volume of the boron nitride the layers stack to form a 3-dimensional network of sheets with little atoms in between the sheets.
Boron nitride molecules are used as an additive to create a harder metal in steels. Before cooling, boron nitride is used by adding it directly to molten steel. Boron Nitride improves the toughness, strength, and ductility of the steel. It does this by increasing the number of free dislocations within the microstructure of the steel. Free dislocations within the microstructure allow for easier deformation of metals, making them more ductile, tougher, and stronger, making them ideal for use as steel additives in industries such as car manufacturing and aircraft manufacturing.
Is boron nitride harmful?
The health application of boron nitride has been examined and has shown no toxic effects on humans or animals. Animals such as rats and guinea pigs exposed to boron nitride for extended periods of time did not show any adverse effects on their bodies in the form of cancer, tumors, or other harmful side effects.
Boron Nitride compound is non-toxic, non-irritating, non-corrosive, non-carcinogenic, non-toxic, and non-allergenic. Due to its strong chemical bonds, boron nitride cannot be dissolved or degraded by solvents.
When was boron nitride discovered?
Boron Nitride was discovered by Italian chemist Eugenio Curatti in 1893 and was named by him “nitridi buturi.” He also predicted that it would occur as a natural mineral in 2002, and ever since then, it has been actively searched for these minerals, but still no natural minerals have yet been found. Since no natural boron nitride has been found, the properties of this compound are synthetic.
How to make boron nitride?
Boron Nitride has been synthesized by a series of reactions involving nitrite, boric acid, and heat. These reactions are carried out at temperatures between room temperature to 1100°C and are carried out in sealed glass or quartz tubes.
Boron Nitride can also be created by directly mixing boric acid with nitric acid and heating the mixture for about two hours at over 2000°C. A series of reactions called metathesis can synthesize the same compounds. Boric oxide and nitric oxide react to form boron nitride and nitrogen dioxide in the metathesis. This reaction is exothermic, meaning that it releases heat. Other reactions such as hydrolysis of boric acid can also be used to synthesize boron nitride. Boron Nitride has also been created through a series of reactions involving potassium chlorate, sodium carbonate, and a silica solution in water.
What is boron nitride used for?
Nitride is used to produce superconductors at low temperatures. Boron Nitride has been used extensively in the development of high-temperature superconductors, in particular, a class of ceramic-based superconductor materials. Boron Nitride has been used as part of the composites in these superconductors. Boron nitride has been found to increase these ceramic-based materials’ critical temperature. These superconductor materials are being studied for their potential use in power transmission lines and nuclear magnetic resonance imaging equipment due to their ability to carry large currents at very low temperatures.
Researchers have found that boron nitride nanotubes can be made from a single layer of boron atoms and are perfectly aligned. This understanding has led to the development of nanofluidic devices with novel properties not seen in existing semiconductor nanowires. The unique properties of these devices include ultra-low power dissipation and excellent thermal conductivity. These key features make them ideal candidates for applications such as heat spreaders and thermoelectric generators.
Boron Nitride is also a very promising biomaterial due to its high chemical stability against attack from acids, bases, and oxidizers, making it an attractive candidate in biologically related applications involving humans.
Boron nitride is being considered a replacement for silicon in transistors and other electronic components of that sort. It is also being used as a replacement for graphene in applications such as flexible electronics, structural components, and microelectromechanical systems (MEMS) because of its good electrical properties and long-term stability at high temperatures.
Boron Nitride has been found to have a catalytic effect on the formation of hydrocarbons from carbon monoxide and hydrogen at high pressures. These molecules have been considered to be key constituents for the formation of life on Earth. Scientists believe that these molecules formed through the process of Metal-Organic Chemistry which involves metal complexes. Boron Nitride is a key substance in this process because it acts as a catalyst in the formation of these molecules. This process is intended to be a way to understand the origin and evolution of life on Earth by recreating similar prebiotic conditions in which these molecules are created. The presence of boron nitride nanotubes was also found in interstellar dust clouds.
The mechanism behind this occurrence is still unknown, but boron nitride nanotubes have been present in the gas phase in the interstellar medium. The study of these dust clouds can be helpful in understanding how life originated on Earth and what role it played. The formation of hydrocarbons in interstellar clouds through the process of Metal-Organic Chemistry is still not very well understood. Still, boron nitride is considered to be key to these reactions taking place. Therefore, it can give an insight into the origin of life on Earth and other planets.
What are the properties of boron nitride?
Boron Nitride has been found as an active coating for cooling systems. Its ability to withstand temperatures up to 1600°C makes it ideal for this application. The anti-reflection characteristics of Boron Nitride make it ideal for use as a coating over optical surfaces. It was first demonstrated in 1998 by NASA researchers on their space telescopes that suffered from some light reflecting off its optics at certain angles. This caused a blurry image on the telescope that could be solved by using Boron Nitride as an anti-reflection layer. It has been used ever since to coat lenses to reduce glare and increase the amount of light transmitted through a lens and onto an object.
Boron Nitride can be synthesized in the form of nanotubes. These nanotubes can be used as electron emitters, light-emitting diodes (LEDs), and lasers. Boron Nitride is also used in the synthesis of doped graphene in order to create a tougher composite material called Graphene-B7N. This composite can be used for medical applications to make better absorbent materials. Boron Nitride has also been used for the fabrication of ultrafast light-emitting diodes (LED) with peak emission at near-infrared wavelengths. The use of Boron Nitride in the fabrication of LEDs is advantageous because it allows the LED to emit almost any wavelength of light instead of using a diode that emits only red light.
Boron Nitride has found in optical fibers and electric capacitors. Boron Nitride is used in the fabrication of electric double-layer capacitors (EDLC) to store electricity. These EDLCs can be used in power applications because they can carry large amounts of charge, have high capacitance, and have a long lifetime.
Boron Nitride has also been used to make microstructures made from layers of boron nitride that are approximately 30 nanometers thick with an upper layer 1,000 times thicker. This layer of boron nitride is about ten times thicker than a single layer of graphene and has much better properties than graphene. The effectiveness of this composite material is shown in its ability to convert infrared light into electric current. Boron Nitride also has an advantage because it can be grown continuously over large areas. In contrast, graphene is not able to be grown over large areas due to limitations caused by its relatively high water solubility.
Boron Nitride has been used as a component in heat spreaders for microprocessor chips and other devices that can reach high temperatures. The greatest feature of using boron nitride as a heat spreader is that it dissipates heat more efficiently than other materials. This makes it ideal for any application requiring high thermal conductivity, such as power generation and transmission and electronics manufacturing.
Heat spreaders are devices that conduct heat from one location to another. These devices can be made from various materials, but boron nitride is used more than the others because of its high thermal conductivity and low thermal expansion coefficient.
What are boron nitride side effects?
The side effects of boron nitride are not documented and are not known. However, some studies indicate that it might increase the risk of cancer because of its long-term exposure to high temperatures.
Boron Nitride has been used for the applications of chemical sensors and electrochemical capacitors. Boron Nitride has been used in sensors to detect gases, such as hydrogen and carbon dioxide, at their parts per million concentrations. The ability to detect these kinds of minute amounts is important because these gases are harmful to living organisms in large enough concentrations. The sensor that has been used to detect these gases can vary in terms of size and efficiency, but the majority of them are at a thickness of only about 50 microns.
Boron Nitride has also been used to make electrochemical capacitors. Electrochemical capacitors work by changing the chemical potential between two electrodes into an electric current through a process known as polarization that is capable of storing large amounts of energy in their plates. Boron Nitride has been used to make electrochemical capacitors because it can store large quantities of charge, has low self-discharge, and is relatively inexpensive.
Boron Nitride has also been used in the synthesis of cathode materials for lithium-ion batteries. These cathode materials are able to store more lithium ions than other materials that are currently being used, such as graphite and silicon. This means that devices will have greater battery life and higher efficiency.
Boron Nitride nanotubes have been seen in interstellar dust clouds, so they have been sought after to be included in spacecraft to serve as potential radiation shields due to their thermal properties. Boron Nitride is considered an ideal candidate for this application because it can withstand very high temperatures and is less susceptible to erosion from extreme temperatures than other materials being used for radiation protection on spacecraft.
Boron Nitride has been used in various applications in the production of automobiles. It has been used in the production of steel because it is a strong, lightweight material that is 30 times harder than silicon carbide and five times harder than titanium. It offers excellent thermal conductivity and can be added to steel at concentrations as high as 30%. The ability to add Boron Nitride to steel makes it ideal for use in automotive applications such as suspension systems, transmission components, and engine cylinder heads.
Boron Nitride has also been used in the production of ceramic piston coatings on automobile engines. These coatings deliver the advantages of strength, hardness, and low thermal expansion. Boron Nitride is one of the few materials that can compete with other materials used in these coatings.
Boron Nitride has been used in various applications in the production of automobiles. It has been used to make disks for use in brakes and suspensions because it has a high stiffness-to-weight ratio and can be coated with a metal such as titanium or steel to improve friction. Boron Nitride also offers better wear resistance than many other materials that are being used for these applications because it does not suffer from metal fatigue as some other materials do.
Boron Nitride has also been used to make power steering components and steering rods. These components offer high stiffness-to-weight ratios, low friction, and high thermal conductivity.
Boron Nitride has been used in various applications in the production of petroleum wells. Oil is pumped from the well by moving down through a pipe that has a lower density than the surrounding water in the ground. A borosilicate tube that is made of Boron Nitride can be used to reduce the amount of friction caused by this movement while still allowing oil to be transported through them. The isotropic properties of Boron Nitride are ideal for this application because the tube will not expand or contract radially, which is a property that does not exist in other materials.
What are boron nitride nanotubes used for?
Boron Nitride nanotubes have been seen in interstellar dust clouds, so they have been sought after to be included in spacecraft to serve as potential radiation shields due to their thermal properties. Boron Nitride is considered an ideal candidate for this application because it can withstand very high temperatures and is less susceptible to erosion from extreme temperatures than other materials being used for radiation protection on spacecraft.
X-ray optics are devices that can be used in many applications, including semiconductors, aerospace engineering, and medicine. Boron Nitride has been used in the production of these optics because it has low absorption in the X-ray spectral region and because it is relatively soft, which can allow it to be easily machined.
Boron Nitride is also able to maintain its properties while dealing with higher temperatures than many other materials and is considered a good candidate for use in these types of applications.
Boron Nitride has also been used in the production of insulators for power transmission lines due to its high melting point, low vapor pressure, and nonreactive characteristics. These characteristics increase power transmission efficiency by keeping power flowing through a line unharmed.
Boron Nitride has been used to make a gamma-ray detector. These detectors are able to detect and identify the sources of gamma-rays by using the rotation of electrons from an excess of photons from a radioactive source. Boron Nitride has been used to make this detector because it is less sensitive to high temperatures and is less susceptible to radiation damage than other materials that were being considered for use in this application.
Boron Nitride has also been used in nuclear reactors, but not for cooling purposes as was first thought. It has been used to load fuel into nuclear reactors because of its high melting point and strength.
Boron Nitride nanotubes have been seen in interstellar dust clouds, so they have been sought after to be included in spacecraft to serve as potential radiation shields due to their thermal properties. Boron Nitride is considered an ideal candidate for this application because it can withstand very high temperatures and is less susceptible to erosion from extreme temperatures than other materials being used for radiation protection on spacecraft.


